All published articles of this journal are available on ScienceDirect.
Dexamethasone Intravitreal Implant for Previously Untreated Macular Edema due to Retinal Vein Occlusion: An Open-label Phase IV Study in China
Abstract
Introduction/objective
Effective treatment of retinal vein occlusion (RVO) is critical to improve vision outcomes. Requirements for repeated intravitreal injections highlight the need for treatments with reduced dosing frequency. The open-label, post-approval YANGTZE study (NCT03908307) evaluated dexamethasone implant in Chinese patients with macular edema due to RVO.
Methods
Eligible patients had previously untreated macular edema due to RVO. Patients received dexamethasone implant 700 μg and were followed up for 12 months; additional injections were administered based on clinical judgement. Primary endpoints were mean change from baseline (CFB) in best-corrected visual acuity (BCVA), proportion of patients with improvement in BCVA ≥15 letters at month 6, and area under the curve (AUC) of average CFB in BCVA.
Results
Overall, 70 patients were enrolled and treated with dexamethasone implant (mean: 2.3 injections). Mean ± standard deviation CFB in BCVA was 10.3 ± 12.1 letters at month 6 and 10.5 ± 12.1 letters at month 12 (both p < 0.001 vs. baseline). Improvement from baseline in BCVA of ≥15 letters was reported in 34.3% and 37.1% of patients at months 6 and 12, respectively. AUC analysis of CFB in BCVA showed significant improvement (p < 0.001). The most frequent adverse event was increased intraocular pressure, reported in 26 patients (37.1%).
Discussion
Despite less frequent administration than typical aVEGF therapy, dexamethasone implant still leads to clinically meaningful improvements in functional and anatomical outcomes.
Conclusion
Dexamethasone intravitreal implant was associated with sustained improvements in BCVA in Chinese patients with RVO. Safety outcomes were consistent with previous studies.
1. INTRODUCTION
Macular edema is defined as swelling in the macular area and is often associated with blurry vision [1, 2]. Various causes have been identified, including diabetic retinopathy, age-related macular degeneration, retinitis pigmentosa, uveitis, retinal vein occlusion (RVO), eye surgery, and certain medications [1, 2]. RVO is the second most common retinal vascular disease and an important cause of vision loss in older patients. It can be divided into two types based on the location of the occluded vein [3]. Central retinal vein occlusion (CRVO) is an occlusion of the main retinal vein, typically caused by thrombosis, and usually affects only one eye [3]. Branch retinal vein occlusion (BRVO) affects the peripheral veins in the retina and is typically caused by arteriosclerotic changes that lead to thrombosis [3, 4].
The goals of treatment for RVO are to reduce macular edema and improve vision. Recommended treatment options for patients with CRVO or BRVO include dexamethasone intravitreal implant and intravitreal injection of anti-vascular endothelial growth factor (aVEGF) therapies, such as ranibizumab, aflibercept, or bevacizumab [5-9]. Laser photocoagulation is also an option for patients with BRVO [5]. An important consideration in the use of intravitreal aVEGF therapy is the frequency of dosing, with available therapies typically administered at 4-week intervals during the initial treatment period [6-9]. Thus, there is a need for treatment options with a longer interval between doses [10].
Dexamethasone intravitreal implant (OZURDEX®) is a sterile, degradable implant that is inserted into the vitreous, offering a therapeutic option with extended dosing intervals [11]. In patients with RVO, a single injection of dexamethasone implant 700 μg has been shown to improve best-corrected visual acuity (BCVA) and reduce central retinal thickness (CRT). In a randomized, double-blind, multicenter, sham-controlled, phase III study of dexamethasone intravitreal implant in patients with macular edema secondary to RVO in China, a single dexamethasone implant provided rapid improvement in BCVA, with improvements in visual and anatomic outcomes maintained for 3 to 4 months after a single implant [12]. Similar results were observed in a prospective, observational, post-marketing surveillance study in South Korea on patients with macular edema following BRVO or CRVO, with the maximum improvement in BCVA observed at 2 months and efficacy maintained in some subgroups for up to 6 months [13]. Re-treatment with a second implant at 6 months in a clinical trial setting has also demonstrated overall similar efficacy and safety to the initial implant, with the notable exception of increased incidence of cataracts [14]. Evaluation of real-world treatment in a post-marketing surveillance study in Europe showed a median dosing interval of 6.2 months [15].
Dexamethasone implant is the first approved intravitreal injection for RVO in China, and is licensed for the treatment of macular edema following BRVO or CRVO [16]. Here, we report a post-approval, real-world study conducted in Chinese clinical settings to evaluate the efficacy and safety of dexamethasone implant 700 μg in previously untreated patients with macular edema due to BRVO or non-ischemic CRVO.
2. MATERIALS AND METHODS
2.1. Study Design
This multicenter, open-label, post-approval study (YANGTZE Study; NCT03908307) was conducted in Chinese clinical settings to evaluate the safety and efficacy of dexamethasone implant 700 μg in previously untreated patients with macular edema due to RVO. Enrolled patients were followed up for 12 months after the first injection, during which time additional injections could be administered based on the investigator’s clinical judgement (Fig. 1).
Dexamethasone implant was administered according to the approved product label in China and performed using a sterile technique. Patients were monitored for the elevation of intraocular pressure (IOP) and endophthalmitis, following each site’s standard of practice. Additional monitoring could include checks for perfusion of the optic nerve head immediately after the injection, tonometry within 30 min following the injection, and biomicroscopy between 2 and 7 days following the injection. Patients were instructed to report any symptoms suggestive of endophthalmitis (eye pain, swollen eyelid, redness of the eye, photophobia, or ocular discharge) without delay [17]. Based on the investigator’s opinion, additional injections were considered for responding patients who demonstrated visual acuity loss. The recommended retreatment interval was no less than 4 months at any point in the study.
This study was conducted in accordance with the Ethical Principles for Medical Research Involving Human Subjects reported in the Declaration of Helsinki. All study-related documents were reviewed and approved by independent ethics committees and/or institutional review boards of each study center (Table S1). All patients signed informed consent forms before entering the study.
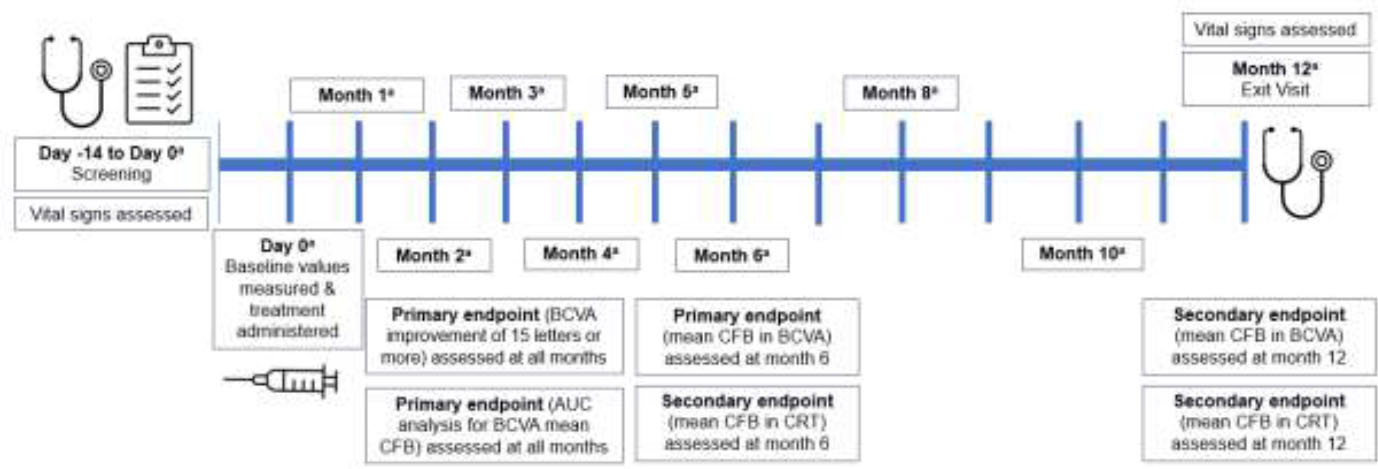
Study design. aBCVA (Early Treatment of Diabetic Retinopathy Study), optical coherence tomography, biomicroscopy, intraocular pressure measurement, and ophthalmoscopy were carried out at all visits. Abbreviations: AUC, area under the curve; BCVA, best-corrected visual acuity; CFB, change from baseline; CRT, central retinal thickness.
2.2. Patients
Included patients were previously untreated men or women ≥ 18 years old, with a diagnosis of macular edema due to BRVO or non-ischemic CRVO of less than 3 months' duration. Patients must have had a retinal thickness ≥ 300 μm as determined by the investigator, assessed by spectral-domain optical coherence tomography in the central 1-mm macular subfield, and a BCVA score of 19 to 73 letters (approximately 20/400 to 20/40 Snellen equivalent) in the treatment eye, measured by Early Treatment Diabetic Retinopathy Study (ETDRS) [18]. Women of childbearing potential provided a negative urine pregnancy test at the baseline visit.
Patients with uncontrolled systemic disease, presence/history of any ocular condition that affected visual acuity other than macular edema, and prior intraocular surgery, laser photocoagulation, intraocular injection, or periocular steroid injection within 3 months prior to study entry were excluded. Patients with a history of IOP elevation in response to steroid treatment that required IOP-lowering treatment, resulted in an increase in IOP > 10 mmHg from pre-dose or resulted in IOP > 25 mmHg were also excluded.
2.3. Study Endpoints
The primary efficacy endpoints were mean change from baseline (CFB; defined as day 0 when the first dose of study medication was administered) in BCVA at the 6th month, the proportion of patients with a BCVA improvement of 15 letters or more compared with baseline, and area under the curve (AUC) of average CFB in BCVA (Fig. 1).
Secondary efficacy endpoints included mean CFB in BCVA after the first follow-up visit, at each injection, and at the 12th month, mean CFB in CRT at months 6 and 12, mean number of injections during the 12-month study, and time to second and third injections. Safety endpoints included the nature, frequency, and severity of adverse events (AEs), including changes in IOP, biomicroscopy, ophthalmoscopy, and vital signs (blood pressure and pulse rate).
2.4. Statistical Analysis
Given that BRVO is 4–6 times more prevalent than CRVO [19], the target enrolment ratio was 4:1 for BRVO:CRVO patients. The enrolled set included all enrolled patients. The safety set included patients who were administered at least one injection. The evaluable set comprised all patients who received one injection and had at least one assessment for the primary efficacy endpoints.
All statistical tests were two-sided hypothesis tests performed at the 5% level of significance for main effects. All confidence intervals (CIs) were two-sided 95% CIs unless stated otherwise. Changes from baseline were analyzed using one-sample t-tests at a 5% level of significance, and the corresponding p-values were reported. All summary tables used only the measurements of the study eye.
Safety outcomes were tabulated by observing the nature, severity, and frequency of AEs throughout the study, as well as changes in vital signs, IOP, biomicroscopy, and ophthalmoscopy. Safety data were summarized descriptively.
Enrollment of 150 treatment eyes was originally targeted to allow for a sample size of 120 eyes, assuming a 20% drop-out rate. The study size was re-evaluated in the context of COVID-19 restrictions to allow for a total enrollment of 70 patients (assuming 54 completers, comprising 43 patients with BRVO and 11 patients with CRVO), which was calculated to provide 85% power to detect a treatment effect for mean ± standard deviation (SD) CFB in BCVA of 3 ± 10 letters.
3. RESULTS
3.1. Demographics and Interventions
In total, 70 patients were enrolled at nine study sites in China and received the first dose of study treatment between July 2019 and October 2020. A total of 64 patients (91.4%) completed 12 months of follow-up, while 6 (8.6%) discontinued before 12 months (three due to physician decision and one each due to an AE of vitreous hemorrhage, patient withdrawal, and unknown reason). The last patient completed the study in September 2021.
The demographics and characteristics of the enrolled population, as well as details of study interventions, are shown in Table 1. In the BRVO subgroup, the median age was 56 years (range 29–83), 24 patients were female (53.3%), and the left eye was studied in 25 patients (55.6%). In the CRVO subgroup, the median age was 57 years (range, 28–74), 13 patients were female (52.0%), and the left eye was studied in nine patients (36.0%). The mean number of injections was 2.2 in the BRVO subgroup and 2.4 in the CRVO subgroup. The overall patient population had a median age of 56 years (range 28–83), and 37 patients were female (52.9%). Primary diagnosis was BRVO in 45 patients (64.3%) and CRVO in 25 patients (35.7%). Patients received a mean of 2.3 dexamethasone implant injections, with 21 (30.0%), 17 (24.3%), 24 (34.3%), and eight (11.4%) patients receiving one, two, three, and four injections, respectively. The mean times from the first to second, second to third, and first to third injection were 137 days, 123 days, and 241 days, respectively.
3.2. Efficacy
3.2.1. Primary Endpoints
BCVA was significantly improved from baseline at month 6 in the overall population, with a mean ± SD CFB of 10.3 ± 12.1 letters (p < 0.001). BCVA improved in both the BRVO and CRVO subgroups, with mean ± SD CFB of 12.0 ± 10.2 letters (p < 0.001) and 7.0 ± 14.8 letters (p = 0.039), respectively (Table 2).
| - | Dexamethasone Intravitreal Implant 700 μg | ||
|---|---|---|---|
| Characteristic |
BRVO (n = 45) |
CRVO (n = 25) |
Overall (N = 70) |
| Age, years | - | - | - |
| Mean ± SD | 56.5 ± 10.2 | 56.2 ± 11.5 | 56.4 ± 10.6 |
| Median (range) | 56.0 (29–83) | 57.0 (28–74) | 56.0 (28–83) |
| Sex, n (%) | - | - | - |
| Male | 21 (46.7) | 12 (48.0) | 33 (47.1) |
| Female | 24 (53.3) | 13 (52.0) | 37 (52.9) |
| Primary diagnosis, n (%) | - | - | - |
| BRVO | - | - | 45 (64.3) |
| CRVO | - | - | 25 (35.7) |
| Ethnicity, n (%) | - | - | - |
| Chinese | 45 (100) | 25 (100) | 70 (100) |
| Study eye, n (%) | - | - | - |
| Left | 25 (55.6) | 9 (36.0) | 34 (48.6) |
| Right | 20 (44.4) | 16 (64.0) | 36 (51.4) |
| Number of injections, n | - | - | - |
| Mean ± SD | 2.2 ± 0.9 | 2.4 ± 1.2 | 2.3 ± 1.0 |
| Median (range) | 2 (1–4) | 3 (1–4) | 2 (1–4) |
| Number of injections, n patients (%) | - | - | - |
| 1 | 13 (28.9) | 8 (32) | 21 (30.0) |
| 2 | 13 (28.9) | 4 (16) | 17 (24.3) |
| 3 | 16 (35.6) | 8 (32) | 24 (34.3) |
| 4 | 3 (6.7) | 5 (20) | 8 (11.4) |
| Time from first injection to second injection, days | - | - | - |
| n | 32 | 17 | 49 |
| Mean ± SD | 136.2 ± 46.1 | 138.6 ± 51.4 | 137.0 ± 47.5 |
| Median (range) | 125 (91–311) | 126 (85–238) | 126 (85–311) |
| Time from second injection to third injection, days | - | - | - |
| n | 19 | 13 | 32 |
| Mean ± SD | 126 ± 32.7 | 119.4 ± 29.0 | 123.3 ± 30.9 |
| Median (range) | 122 (86–210) | 119 (86–180) | 119 (86–210) |
| Time from first injection to third injection, days | - | - | - |
| n | 19 | 13 | 32 |
| Mean ± SD | 245.5 ± 41.7 | 234.4 ± 47.8 | 241.0 ± 43.9 |
| Median (range) | 249 (184–308) | 241 (182–308) | 245 (182–308) |
| - | Dexamethasone Intravitreal Implant 700 μg | ||
|---|---|---|---|
| Outcome |
BRVO (n = 45) |
CRVO (n = 25) |
Overall (N = 70) |
| Baseline | |||
| n | 45 | 25 | 70 |
| Mean ± SD | 57.8 ± 12.3 | 54.2 ± 14.3 | 56.5 ± 13.1 |
| Median (range) | 62.0 (23 to 73) | 59.0 (23 to 72) | 60.0 (23 to 73) |
| 6 months | |||
| n | 42 | 22 | 64 |
| Mean ± SD | 69.5 ± 13.5 | 62.1 ± 19.7 | 66.9 ± 16.1 |
| Median (range) | 73.5 (31 to 87) | 69.0 (23 to 86) | 72.5 (23 to 87) |
| CFB | - | - | - |
| Mean ± SD | 12.0 ± 10.2 | 7.0 ± 14.8 | 10.3 ± 12.1 |
| 95% CIa | (8.8 to 15.2) | (0.4 to 13.5) | (7.3 to 13.3) |
| H0: Mean change = 0, pa | < .001 | .039 | < .001 |
| Median (range) | 12.0 (–7 to 32) | 9.5 (−34 to 24) | 11.5 (−34 to 32) |
| Improved patientsb, n (%) | - | - | - |
| 1–5 letters | 6 (13.3) | 2 (8.0) | 8 (11.4) |
| 6–10 letters | 6 (13.3) | 4 (16.0) | 10 (14.3) |
| 11–15 letters | 8 (17.8) | 2 (8.0) | 10 (14.3) |
| 15 letters or more | 16 (35.6) | 8 (32.0) | 24 (34.3) |
| 12 months | |||
| n | 42 | 22 | 64 |
| Mean ± SD | 69.5 ± 13.1 | 61.8 ± 19.3 | 66.9 ± 15.8 |
| Median (range) | 71.0 (39 to 88) | 66.5 (22 to 88) | 70.5 (22 to 88) |
| CFB | - | - | - |
| Mean ± SD | 11.6 ± 11.2 | 8.5 ± 13.7 | 10.5 ± 12.1 |
| 95% CIa | (8.1 to 15.1) | (2.4 to 14.5) | (7.5 to 13.5) |
| H0: Mean change = 0, pa | < .001 | .009 | < .001 |
| Median (range) | 10.5 (−7 to 35) | 10.0 (−18 to 28) | 10.0 (−18 to 35) |
| Improved patientsb, n (%) | - | - | - |
| 1–5 letters | 10 (22.2) | 3 (12.0) | 13 (18.6) |
| 6–10 letters | 5 (11.1) | 3 (12.0) | 8 (11.4) |
| 11–15 letters | 7 (15.6) | 1 (4.0) | 8 (11.4) |
| 15 letters or more | 16 (35.6) | 10 (40.0) | 26 (37.1) |
Improvement in BCVA of 15 letters or more was reported in 34.3% of patients in the overall population at the month 6 and 37.1% of patients at the month 12. Improvements in the BRVO and CRVO subgroups were consistent with the overall population, with improvement in BCVA of 15 letters or more in 35.6% of patients in the BRVO subgroup and 32.0% of patients in the CRVO subgroup at month 6 and 35.6% and 40.0%, respectively, at month 12. Overall, AUC for mean CFB in BCVA increased throughout the study, with significant differences from baseline at all time points (all p < 0.001).
3.2.2. Secondary Endpoints
The mean ± SD CFB in BCVA in the overall population, BRVO subgroup, and CRVO subgroup at the month 12 was 10.5 ± 12.1, 11.6 ± 11.2, and 8.5 ± 13.7, respectively (Table 2). Increases from baseline were statistically significant at all time points from 1 month in both the BRVO and CRVO subgroups (all p < 0.05), except for the month 3 in the CRVO subgroup (p = 0.304). The magnitude of increase from baseline was slightly higher in the BRVO subgroup than in the CRVO subgroup from month 3 onward (Fig. 2A).
The mean ± SD CFB in CRT in the overall population was −236.5 ± 221.6 μm at month 6 and −290.5 ± 208.2 μm at month 12. Although fluctuations were observed, mean CFB in CRT ranged from −208.8 μm to −355.4 μm throughout the study. Reductions from baseline in CRT were significant at all time points (all p < 0.001; Fig. 2B).
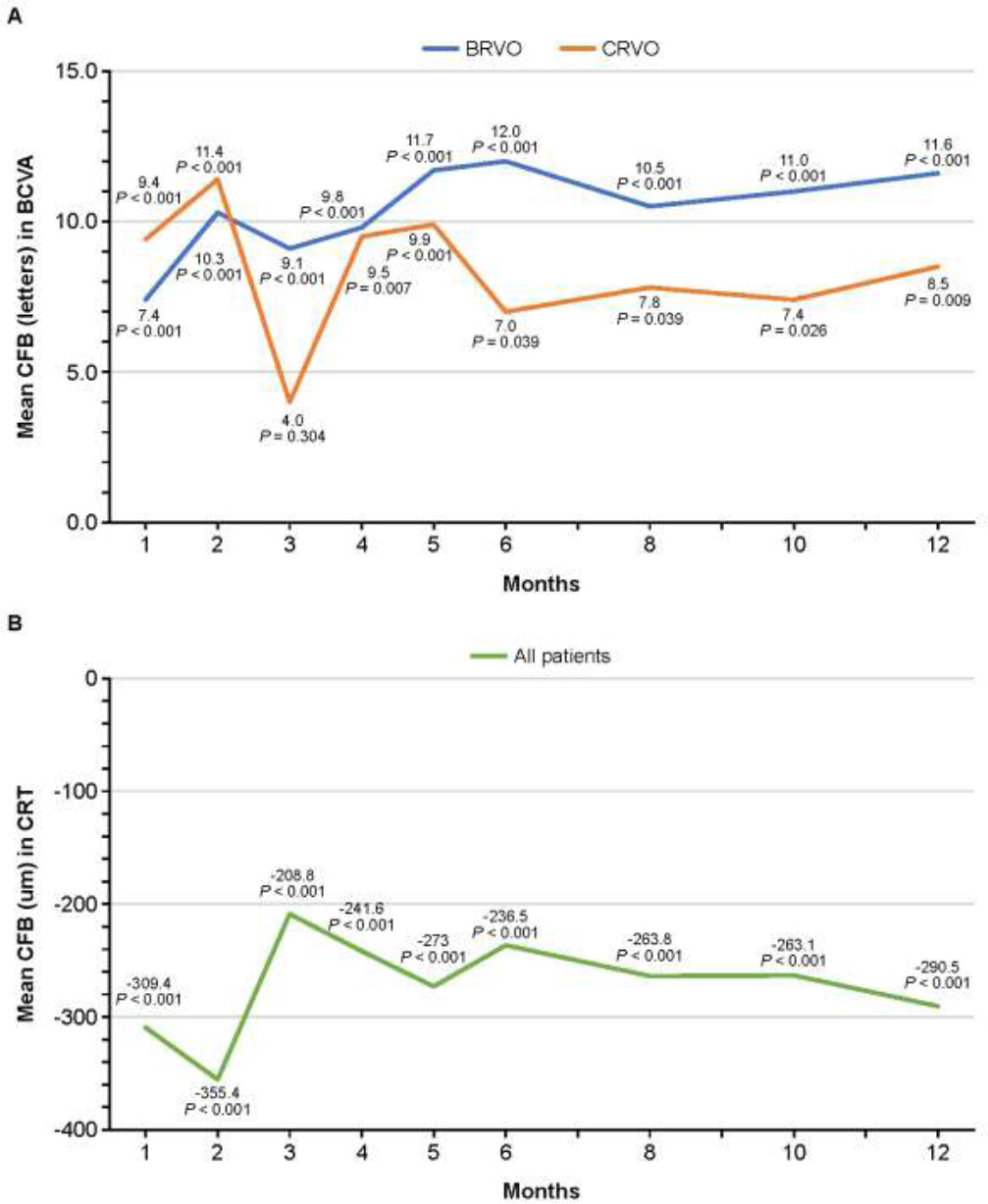
Mean CFB in (A) BCVA and (B) CRT over 12 months. Abbreviations: BCVA, best-corrected visual acuity; BRVO, branch retinal vein occlusion; CFB, change from baseline; CRT, central retinal thickness; CRVO, central retinal vein occlusion.
| System Organ Class and Preferred Term |
Dexamethasone Intravitreal Implant 700 μg (N = 70) |
|
|---|---|---|
| Patients, n (%) | Events, n | |
| Any TEAE related to study treatment | 33 (47.1) | 69 |
| Investigations | 26 (37.1) | 42 |
| Intraocular pressure increased | 26 (37.1) | 42 |
| Eye disorders | 16 (22.9) | 27 |
| Conjunctival hemorrhage | 4 (5.7) | 5 |
| Cataract | 4 (5.7) | 4 |
| Posterior capsule opacification | 4 (5.7) | 4 |
| Cataract nuclear | 3 (4.3) | 3 |
| Ocular hypertension | 2 (2.9) | 3 |
| Macular fibrosis | 2 (2.9) | 2 |
| Asthenopia | 1 (1.4) | 1 |
| Conjunctival hyperemia | 1 (1.4) | 1 |
| Dry eye | 1 (1.4) | 1 |
| Macular hole | 1 (1.4) | 1 |
| Ocular hyperemia | 1 (1.4) | 1 |
| Xerophthalmia | 1 (1.4) | 1 |
3.3. Safety
Overall, AEs were reported in 49 patients (70.0%). Treatment-emergent AEs (TEAEs) were reported in 48 patients (68.6%), including injection-site TEAEs in 29 patients (41.4%). TEAEs were considered to be treatment-related in 33 patients (47.1%). In total, 132 TEAEs were reported during the study, most of which were mild (90/132 events) or moderate (36/132 events), with six severe TEAEs reported in five patients. One patient (1.4%) discontinued treatment due to a TEAE of vitreous hemorrhage. Of the 132 TEAE events reported, 103 resolved without sequelae. At the time of the last follow-up, 22 events were unresolved, of which eight were treatment-related (three nuclear cataracts, two cataracts, two increased IOP, and one posterior capsule opacification). Further, six events were resolved, and one event was resolved with sequelae.
The most common TEAEs were increased IOP (26 patients, 37.1%), conjunctival hemorrhage, and dry eye (both five patients each, 7.1%). IOP was the most common treatment-related TEAE, with all events considered to be related to study treatment. TEAEs of cataract or nuclear cataracts, were also reported in seven patients (10.0%) (Table 3). Seven patients (10.0%) experienced at least one injection-related TEAE, the most common being conjunctival hemorrhage (five patients, 7.1%). A total of 10 serious TEAEs were reported in seven patients (10.0%), with four events in two patients considered to be related to study treatment (cataract in one patient who had received four injections of study treatment, and cataract, macular fibrosis, and macular hole in a patient who had received a single injection of study treatment). No notable changes in vital sign parameters were reported during the study.
Dexamethasone implant was associated with significant increases from baseline in mean IOP at all time points (all p < 0.05; range 0.99 mmHg to 5.13 mmHg). No notable changes in ophthalmoscopy or biomicroscopy findings were observed during the study period.
4. DISCUSSION
Macular edema due to RVO has an estimated prevalence in China of 0.5%, with the highest prevalence in elderly people (2.1% and 2.8% in patients aged 60–69 and ≥ 70 years, respectively) [20, 21]. Inadequate treatment of RVO may have a significant negative impact on patient outcomes, and the need for multiple intravitreal injections is a potential barrier during the early stages of treatment.
In this real-world study in Chinese patients with macular edema due to RVO, dexamethasone intravitreal implant was associated with sustained improvements in BCVA and CRT and was well tolerated. Mean BCVA at months 6 and 12 was significantly improved from baseline in the overall study population (both p < 0.001), as well as in subgroups with BRVO (both p < 0.001) and CRVO (p < 0.039 and p < 0.01, respectively). Significant improvement from baseline in BCVA was reported at all time points throughout the study in both the BRVO and CRVO subgroups, with the exception of month 3 in the CRVO subgroup. A total of 34.3% of patients demonstrated an improvement in BCVA of 15 letters or more in the month 6, which was maintained at the month 12, with an improvement of 15 letters or more reported in 37.1% of patients. The proportion of patients with an improvement in BCVA of 15 letters or more was consistent across both the BRVO and CRVO subgroups. The AUC for mean change in BCVA increased throughout the study, with significant increases from baseline at all visits (p< 0.001). Significant reductions in CRT were also observed, with a mean CFB of −290.5 μm at month 12.
The outcomes observed in the present analysis are consistent with previous reports of clinical trials and observational studies of dexamethasone intravitreal implants [12, 13]. In a phase III study in patients with macular edema secondary to RVO in China, a single injection of dexamethasone implant provided rapid improvement in BCVA, with a CFB of 15 letters or more in 35% of patients and a mean ± SD improvement of 10.6 ± 10.4 letters in the month 12 [12]. Similarly, dexamethasone implant improved BCVA in patients with macular edema due to RVO in a post-marketing surveillance study in South Korea [13].
Available data suggest that dexamethasone implant allows for less frequent dosing than intravitreal aVEGF therapy, while still achieving clinically meaningful improvement in functional and anatomical outcomes [6-9]. In the current study, patients received a mean of 2.3 ± 1.0 injections over the 12-month study period, with 88.6% of patients receiving three injections or fewer. By contrast, studies evaluating as-needed dosing of aflibercept and ranibizumab suggest more frequent dosing than reported for dexamethasone implant. In the 1-year GALILEO study, patients receiving six doses of aflibercept at 4-weekly intervals followed by as-needed dosing received a mean total of 11.8 injections [22]. In the 1-year COPERNICUS study, patients received a mean of 2.7 injections of aflibercept during 28 weeks of as-needed treatment [23]. In the 1-year CRYSTAL study, patients receiving three doses of ranibizumab at 4-weekly intervals followed by as-needed treatment received a mean total of 8.1 injections [24]. Finally, in the 1-year CRUISE study, patients received a mean of 3.8 and 3.3 injections of ranibizumab 0.3 mg and 0.5 mg, respectively, during 6 months of as-needed treatment [25].
The long-term efficacy of dexamethasone implant and intravitreal aVEGF injection in patients with macular edema secondary to RVO were compared in a small (n = 16), 5-year, retrospective real-world study, which found that both treatment options had similar long-term effects on visual acuity. Patients in the dexamethasone group received one implant for a minimum period of 6 months before re-evaluation, while patients in the aVEGF group received monthly injections of ranibizumab or conbercept for 3 months, followed by as-needed treatment. At the 5-year follow-up, BCVA did not differ significantly between the dexamethasone and aVEGF groups (logMAR 0.69 ± 0.36 vs 0.57 ± 0.30, respectively; p = 0.574), and CRT was similar in both groups [26]. These data suggest that dexamethasone implant provided long-term improvement in retinal perfusion, but should be interpreted with caution owing to the limited sample size [26]. Dexamethasone implant has also shown beneficial effects in patients with deterioration in visual acuity on aVEGF therapies in a retrospective case series, with dexamethasone implant resulting in vision gains at 30 days [27].
The safety profile of dexamethasone implants in the current study was consistent with previous reports, with no unexpected AEs [12, 13, 15]. In a randomized phase III trial in China, the most common TEAE was increased IOP (29.5%), similar to the rate reported in the current study [12]. Increased IOP was also the most frequent AE in the South Korean post-marketing surveillance study, reported in 5.3% of patients [13]. A 2-year observational post-marketing study in patients with macular edema associated with RVO or non-infectious posterior segment uveitis in Europe did not identify any new safety concerns following extended treatment, with a low incidence of serious ocular AEs in treated eyes (2.4% of patients with RVO), the most common being cataract. Increased IOP was reported in 20.4% of patients with RVO and was considered to be a serious AE in one patient. Repeated treatment in the same eye was not associated with any new safety concerns [15].
Cataract development is a known AE associated with increasing dose and duration of corticosteroid treatment [11-14]. In the current study, TEAEs of cataract or nuclear cataract were reported in seven patients (10.0%), with all events considered to be treatment-related; one patient had received a single injection of study treatment, three patients had received three injections, and three patients had received four injections. Cataract TEAEs were reported in two patients (0.9%) in a 6-month randomized phase III trial in China and in a single patient (0.1%) in the South Korean post-marketing surveillance study [12, 13]. In a 2-year post-marketing surveillance study in Europe, cataract formation and progression were reported in 20.0% and 19.2% of patients in the overall population, and patients who received more than two dexamethasone injections had a higher incidence of cataract formation compared with patients who had received one or two injections (24.7% vs. 17.7% baseline phakic eyes) [15]. In a 2-year observational study of patients who received more than two dexamethasone implants, cataract was the most frequently reported AE, occurring in 53.9% (96/178) baseline phakic eyes [11].
This study is subject to several limitations, most notably the reduction in the planned patient enrolment from 150 to 70 patients due to COVID restrictions. However, the 64 patients who completed the study exceeded the estimate of 54 patients used in the revised statistical assumptions. The study was also limited to a 12-month follow-up period, and evaluation of treatment durations of 24 months or longer in future studies would be useful to inform clinical practice.
CONCLUSION
In conclusion, this real-world clinical study demonstrated the efficacy and safety of dexamethasone intravitreal implant 700 μg in previously untreated Chinese patients with macular edema due to RVO. Patients demonstrated sustained improvement in visual acuity for up to 1 year, and the observed safety profile was consistent with previous reports, with most patients requiring two implants or fewer.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: P.W., J.M.: Study conception and design; K.L., W.C., Y.C., Y.C., M.H., W.L., X.M., W.W., H.X., X.X.: Data collection; D.O., P.W., J.J.: Analysis and interpretation of results; D.O., P.W., J.M.: Draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| BCVA | = Best-corrected visual acuity |
| CFB | = Change from baseline |
| CRVO | = Central retinal vein occlusion |
| aVEGF | = Anti-vascular endothelial growth factor |
| CRT | = Central retinal thickness |
| IOP | = Intraocular pressure |
| ETDRS | = Early Treatment Diabetic Retinopathy Study |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All study-related documents were reviewed and approved by independent ethics committees and/or institutional review boards of each study center. The study was approved by the ethics committee of Shanghai General Hospital with approval number 2019[244].
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
All patients signed informed consent forms before entering the study. Written consent was obtained from the study participants.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study can be requested. For more information, please visit the following link: https://vivli.org/ourmember/abbvie/.
CONFLICT OF INTEREST
Financial arrangements of the authors with companies whose products may be related to the present report are listed as declared by the authors: WW, KL, WC, Y.Ch, Y.Cu, MH, WL, XX, HX, and XM have nothing to disclose. DO, JM, and PW are employees of AbbVie and may hold AbbVie stock.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the support provided by Sam Ffrench-Mullen and Tina Allen, BSc, of Spark (a division of Prime, New York, USA), funded by AbbVie, USA.


