All published articles of this journal are available on ScienceDirect.
One Intravitreal Dexamethasone Implant versus Single or Multiple Intravitreal Triamcinolone Acetonide Injections for Macular Edema due to Central Retinal Vein Occlusion: Efficacy and Safety
Abstract
Purpose
The study aimed to compare the efficacy and safety of single or multiple intravitreal triamcinolone acetonide (ITA) injections compared to a single intravitreal dexamethasone implant (IDI) for the treatment of macular edema associated with central retinal vein occlusion (CRVO).
Methods
Between January 2016 and January 2023, a retrospective study was performed on a total of 60 consecutive eyes, with 30 eyes receiving ITA and the other 30 receiving IDI. Best corrected visual acuity (BCVA), central retinal thickness (CRT), intraocular pressure (IOP), and cataract progression were assessed over a follow-up period of 6 months.
Results
Both ITA and IDI groups showed initial improvements in BCVA and CRT at one month, with no significant difference between the groups. However, at six months, there were no notable disparities in BCVA, CRT, IOP increase, or cataract progression between the two treatments. In the ITA group, BCVA improved from baseline to one month and remained stable until six months. The IDI group showed initial improvement but did not display further significant progress at the six-month mark. No statistically significant difference was found between the groups. Retinal thickness decreased significantly in both groups from baseline to one month and continued to improve until six months, with no significant difference between ITA and IDI. Regarding complications, both groups had similar occurrences of transient IOP increases (40% in each group) and cataract progression (40% in ITA, 20% in IDI).
Conclusion
At the final follow-up, no statistically significant differences were observed between the two groups in BCVA, CRT, IOP increase, or cataract progression. Despite the cost difference between the two injections, both treatments can be used with similar efficacy and safety profiles.
1. INTRODUCTION
Macular edema (ME) is caused by a disruption of the blood-retinal barrier, leading to leakage of fluid that accumulates in the retinal layers, resulting in an expansion of the extracellular and/or intracellular spaces of the retina [1]. It occurs in many intraocular and systemic diseases, such as retinal vein occlusion, Irvine-Gass syndrome, diabetic retinopathy, and uveitis, leading to permanent visual loss [1]. The pathogenesis of ME is hypothesized to be due to increased hydrostatic pressure, decreased oncotic pressure, the presence of inflammatory cytokines, and increased permeability factors [1].
One of the leading causes of macular edema is central retinal vein occlusion (CRVO). CRVO occurs when the central retinal vein is occluded by a thrombus near the lamina cribrosa [2]. It has a prevalence of 0.08%, and the risk factors of CRVO include age, hypertension, open-angle glaucoma, and hyperlipidemia. Moreover, it has detrimental effects on the eye, including intraretinal hemorrhage, macular edema, neovascularization, macular ischemia, and subretinal fluid [2]. Treatment options for CRVO presenting with macular edema include intravitreal anti-VEGF and corticosteroids. Anti-VEGF is considered first line and includes ranibizumab, bevacizumab, and aflibercept [2].
Intravitreal triamcinolone acetonide (ITA) has been used in the treatment of macular edema associated with CRVO [2]. It is a crystalline steroid known to have a long-lasting concentration in the eye, up to one month [3]. Another drug used is intravitreal dexamethasone, known as intravitreal dexamethasone implant (IDI), which is given in the form of an intravitreal implant introduced through the pars plana by an applicator. The IDI is made from a biodegradable copolymer that has a sustained release for up to 6 months, with peak response at 60 days. Doses available are 0.35mg and 0.7mg [4]. Mishra et al. compared the two drugs in patients with macular edema related to CRVO and found no statistically significant difference in BCVA or CRT at 6 months [5].
Given the significantly higher cost of the intravitreal dexamethasone implant (IDI) compared to intravitreal triamcinolone acetonide (ITA) and the severe socioeconomic collapse affecting Lebanon since October 2019, this study aimed to assess whether ITA could serve as a safe and effective alternative to IDI despite the price disparity. Lebanon, once considered a medical hub in the Middle East, has been severely destabilized by an ongoing multifaceted crisis. Since late 2019, the country has experienced a historic economic meltdown, characterized by a hyperinflation rate of 154.8%, a drastic devaluation of the local currency, and one of the lowest minimum wages in the world. The situation worsened following the catastrophic Beirut port explosion on August 4th, 2020, one of the most powerful non-nuclear explosions in history, which killed over 200 people, injured more than 6,000, and displaced thousands. As a result, a large proportion of the population has been left struggling to meet basic needs, including food, shelter, and medical care. In this context, patients are often forced to prioritize survival essentials over medical treatments, such as intravitreal injections that cost thousands of dollars [6]. This economic hardship raises a critical question: could a more affordable alternative, such as ITA, deliver comparable outcomes without increasing the risk of complications? This study was, therefore, designed to evaluate the efficacy and safety of ITA as a potential substitute for IDI under these extreme conditions. This study aims to evaluate the efficacy and safety of single or multiple intravitreal injections of triamcinolone acetonide (ITA) and a single intravitreal dexamethasone implant (IDI) for macular edema associated with central retinal vein occlusion in a sample of Lebanese patients.
2. METHODS
2.1. Study Design
This retrospective study involved eyes with macular edema associated with CRVO. Patients received treatment with either ITA or IDI at the Eye and Ear Hospital International in Lebanon, between January 2016 and January 2023. The study included a total of 60 eyes, with 30 eyes receiving ITA and 30 eyes receiving IDI. Inclusion criteria included a follow-up period of 6 months, the presence of CRVO, and treatment with one or multiple ITA injections or a single IDI. Patients with diabetic retinopathy, aphakia, and those treated with subtenon or subconjunctival ITA injections were excluded from the study. Moreover, patients who were given a combination of steroid injections and anti-VEGF injections were excluded from the study. Cataract progression was considered when the cataract reached grade 2 on the Lens Opacities Classification System (LOCS) III. Cataract surgery was performed when the cataract reached grade 3 or higher. IOP spike was considered when an increase of more than 10 mmHg from baseline was observed, or when IOP was more than 21 mmHg.
Ethical approval was granted by the Eye and Ear University Hospital's ethics committee before starting the study. The Declaration of Helsinki's principles were adhered to throughout the study's execution. Prior to using their medical data in our research, all patients were informed and signed a consent form.
2.2. Minimum Sample Size
The G-power software calculated a minimum sample of 20 participants to have enough statistical power based on an alpha error of 5%, a beta error of 20%, and an IOP value of 17.00 ± 1.94 in the dexamethasone group vs 22.2 ± 4.93 in the triamcinolone group according to a previous study (5).
2.3. Patient Data
Patients’ past ocular history included cataract surgery (simple or complicated), prior pars plana vitrectomy, treatment for glaucoma either medically or surgically, presence of rubeosis, retinal detachment, or macular hole. Post-injection complications were observed, along with their corresponding treatment: endophthalmitis, elevated intraocular pressure (IOP) exceeding 21 mm Hg, progression of cataracts, intravitreal hemorrhage, epiretinal membrane, retinal detachment, flare-up of herpetic keratitis, and corneal decompensation.
Outcome measurements were conducted to assess the results, which included best-corrected visual acuity (BCVA) measured using a Snellen chart (converted to logMAR for statistical purposes), central retinal thickness (CRT) measured using spectral domain optical coherence tomography (SD-OCT) in micrometers (µm), and IOP measured with a Goldman applanation tonometer in mmHg. The follow-up protocol was day 1, 1 week, 2 weeks, 1 month, and then monthly until 6 months.
2.4. Intravitreal Injection
All procedures were performed in the minor surgery room under local anesthesia (using topical anesthetic drops) with strict adherence to sterility precautions. A sterile eyelid speculum was used to retract the eyelids before the injection, which was performed 3.5–4 mm from the limbus in the inferotemporal quadrant, as measured with a sterile caliper. Either 0.7 mg of intravitreal dexamethasone implant (IDI) or 0.1 mL of 4 mg intravitreal triamcinolone acetonide (ITA) was administered.
2.5. Anatomical and Functional Success
Anatomical results were considered successful when there was improvement or stabilization of the BCVA and an improvement in the central retinal thickness within the normal range of CRT (below 310 µm) at the final follow-up visit.
2.6. Criteria for Re-injection of ITA
If any of the following criteria were present at least 1 month after the injection, re-injection was considered: residual macular oedema and a decrease in BCVA by 3 or more lines compared to the initial visit.
2.7. Statistical Analysis
IBM SPSS Statistics software version 27 was used for all statistical analyses. Chi-square/Fisher's exact tests were carried out to compare two qualitative variables. The Student's t-test was conducted to compare two means. A repeated-measures ANOVA was used to compare BCVA, CRT, and IOP values at baseline, 1 month, and 6 months. The result was considered statistically significant if the p-value was < 0.05.
3. RESULTS
A total of 60 eyes were included in the study, comprising 31 right eyes (51.67%) and 29 left eyes (48.33%). Thirty eyes received intravitreal triamcinolone acetonide (ITA), and thirty received intravitreal dexamethasone implant (IDI). There were no significant differences between the two groups at baseline regarding age, gender, diabetes, hypertension, or dyslipidemia. Additionally, the number of anti-VEGF injections administered prior to steroid treatment was comparable between groups (p = 0.470) (Table 1).
| - | ITA | IDI | p | Total |
|---|---|---|---|---|
| Characteristics of the patients | ||||
| Age (years) | 63.9 ± 21.49 | 68.2 ± 10.32 | 0.971 | 66.05 ± 16.55 |
| Sex | - | - | 0.650 | - |
| Males | 16 (53.33%) | 15 (50.0%) | - | 31 (51.67%) |
| Females | 14 (46.67%) | 15 (50.0%) | - | 29 (48.33%) |
| Diabetes | - | - | 0.472 | - |
| No | 27 (90%) | 24 (80.0%) | - | 51 (85.0%) |
| Yes | 3 (10%) | 6 (20.0%) | - | 9 (15.0%) |
| Dyslipidemia | - | - | 0.231 | - |
| No | 24 (80%) | 21 (70.0%) | - | 45 (75.0%) |
| Yes | 6 (20%) | 9 (30.0%) | - | 15 (25.0%) |
| Smoking | - | - | 0.313 | - |
| No | 12 (40.0%) | 18 (60.0%) | - | 30 (50.0%) |
| Yes | 18 (60.0%) | 12 (40.0%) | - | 30 (50.0%) |
| Hypertension | - | - | 1 | - |
| No | 9 (30.0%) | 9 (30.0%) | - | 18 (30.0%) |
| Yes | 21 (70.0%) | 21 (70.0%) | - | 42 (70.0%) |
| Coronary artery disease | - | - | 0.158 | - |
| No | 18 (60.0%) | 24 (80.0%) | - | 42 (70.0%) |
| Yes | 12 (40.0%) | 6 (20.0%) | - | 18 (30.0%) |
| Past Ocular History | ||||
| Cataract Surgery | - | - | 0.472 | - |
| No | 27 (90.0%) | 24 (80.0%) | - | 51 (85.0%) |
| Yes | 3 (10.0%) | 6 (20.0%) | - | 9 (15.0%) |
| Glaucoma | - | - | 0.237 | - |
| No | 30 (100%) | 27 (90.0%) | - | 57 (95.0%) |
| Yes | 0 (0%) | 3 (10.0%) | - | 3 (5.0%) |
| Previous injection of anti-VEGF | - | - | 0.470 | - |
| No | 15 (50.0%) | 12 (40.0%) | - | 27 (45.0%) |
| Yes | 15 (50.0%) | 18 (60.0%) | - | 33 (55.0%) |
| Complications | ||||
| Cataract progression | - | - | 0.158 | - |
| No | 18 (60.0%) | 24 (80.0%) | - | 42 (70.0%) |
| Yes | 12 (40.0%) | 6 (20.0%) | - | 18 (30.0%) |
| Intraocular pressure spikes | - | - | 1 | - |
| No | 18 (60.0%) | 18 (60.0%) | - | 36 (60.0%) |
| Yes | 12 (40.0%) | 12 (40.0%) | - | 24 (40.0%) |
3.1. BCVA Results
At the final follow-up, both groups demonstrated improvement in BCVA compared to baseline. In the ITA group, BCVA improved significantly from 0.89 ± 0.55 logMAR at baseline to 0.57 ± 0.60 at 1 month (p < 0.001; 95% CI for the difference [0.19; 0.45]) and remained stable at 6 months with a value of 0.58 ± 0.59 (p = 1; 95% CI for the difference [-0.08; 0.05] vs. 1 month; p < 0.001; 95% CI for the difference [0.18; 0.44] vs. baseline). In the IDI group, BCVA improved significantly from 0.97 ± 0.52 at baseline to 0.86 ± 0.55 at 1 month (p < 0.001; 95% CI for the difference [0.06; 0.16]), and at 6 months, BCVA decreased further (0.76 ± 0.68; 95% CI for the difference [0.06; 0.37]) compared to baseline (p < 0.001), but this change was not significant compared to the 1-month value (p = 0.412; 95% CI for the difference [-0.07; 0.27]) (Fig. 1). Between-group comparisons showed no statistically significant differences in BCVA at baseline (0.89 ± 0.55 vs. 0.97 ± 0.52, the mean difference was -0.82 logMar, 95% CI [-0.60 to 0.44], p=0.556), at 1 month (0.57 ± 0.6 vs. 0.86 ± 0.55, the mean difference was -0.29 logMar, 95% CI [-0.86 to 0.27], p = 0.054), or at 6 months (0.58 ± 0.59 vs. 0.76 ± 0.68, the mean difference was -0.18 logMar, 95% CI [-0.79 to 0.44], p = 0.287).
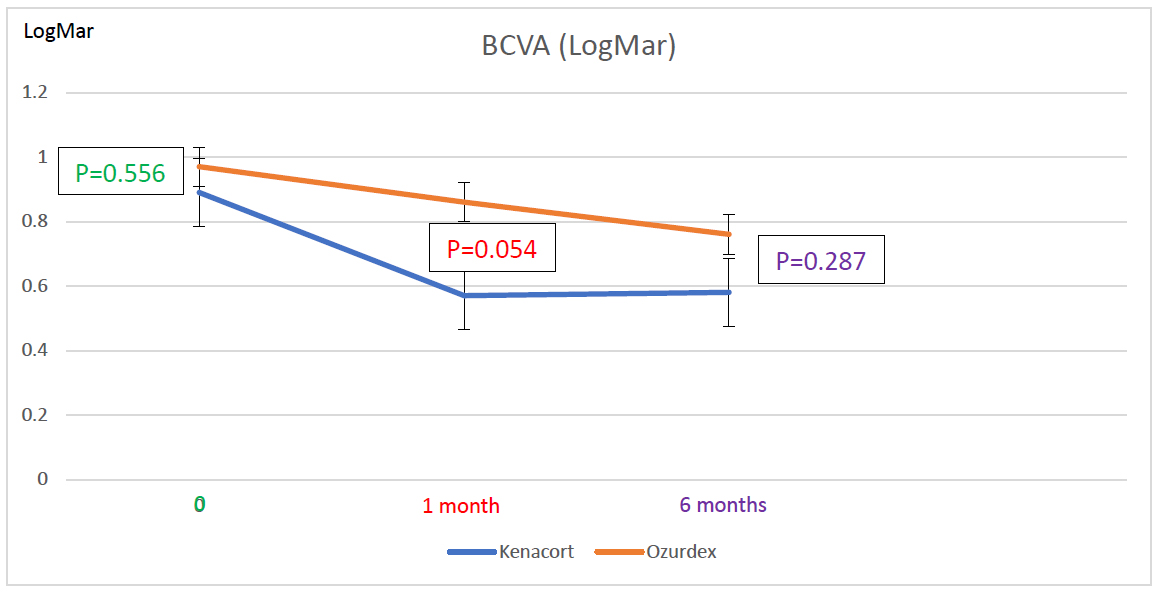
At the initial visit, BCVA was 0.89 ± 0.55 LogMar in the ITA group and 0.97 ± 0.52 LogMar in the IDI group (p = 0.556). At 1month post-injection, BCVA was 0.57 ± 0.6 LogMar in the ITA group and 0.86 ± 0.55 LogMar in the IDI group (p = 0.054). At the final follow-up visit, BCVA was 0.58 ± 0.59 LogMar in the ITA group and 0.76 ± 0.68 LogMar in the IDI group (p = 0.287).
3.2. CRT Results
In the ITA group, CRT also decreased significantly from 463.70 ± 106.59 µm at baseline to 369.70 ± 75.83 µm at 1 month (p < 0.001; 95% CI for the difference [62.86; 125.14]) and to 349.80 ± 83.56 µm at 6 months (p = 0.128; 95% CI for the difference [-3.94; 43.74] vs. 1 month; p < 0.001; 95% CI for the difference [-125.14; -62.86] vs. baseline). In the IDI group, CRT decreased significantly from 473.70 ± 98.69 µm at baseline to 452.60 ± 98.04 µm at 1 month (p < 0.001; 95% CI for the difference [12.83; 29.37]), and further to 405.70 ± 106.91 µm at 6 months (p < 0.001; 95% CI for the difference [20.04; 73.76] vs. 1 month; p < 0.001; 95% CI for the difference [40.15; 95.85] vs. baseline) (Fig. 2). Between groups, CRT was comparable at baseline (463.70 ± 106.59 µm vs. 473.70 ± 98.69 µm; mean difference = -10.0 µm, 95% CI [-110.02 to 90.02], p = 0.708). At 1 month, CRT was significantly lower in the ITA group (369.70 ± 75.83 µm vs. 452.60 ± 98.04 µm, the mean difference was -82.90 µm, 95% CI [-168.24 to 2.44], p < 0.001), while at 6 months, the difference was statistically significant in favor of a lower CRT in the ITA group (349.8 ± 83.56 µm vs. 405.70 ± 106.91 µm, the mean difference was -55.90 µm, 95% CI [-149.33 to 37.53], p = 0.028) (Fig. 2).
3.3. IOP Results
In the ITA group, IOP increased from 14.60 ± 1.22 mmHg at baseline to 17.60 ± 6.70 mm Hg at 1 month (p = 0.038; 95% CI for the difference [-5.86; -0.14]), then decreased significantly to 15.30 ± 4.43 mm Hg at 6 months (p < 0.001; 95% CI for the difference [0.97; 3.64] vs. 1 month; p = 1; 95% CI for the difference [-2.60; 1.20] vs baseline). In the IDI group, IOP increased from 15.60 ± 3.19 mmHg at baseline to 18.70 ± 8.46 mmHg at 1 month but this was not significant (p = 0.063; 95% CI for the difference [-6.33; 0.13]), then decreased to 16.10 ± 4.42 mmHg at 6 months (p = 0.377; 95% CI for the difference [-1.59; 6.79] vs. 1 month and p = 1; 95% CI for the difference [-2.10; 1.10] vs baseline) (Fig. 3). Between-group comparisons showed no significant differences in IOP at baseline (14.6 ± 1.22 mmHg vs. 15.60 ± 3.19 mmHg, the mean difference was -1.0 mmHg, 95% CI [-3.35 to 1.35], p = 0.117), at 1 month (17.6 ± 6.7 mmHg vs. 18.70 ± 8.46 mmHg, the mean difference was -1.1 mmHg, 95% CI [-8.53 to 6.33], p = 0.579), or at 6 months (15.3 ± 4.6 mmHg vs. 16.10 ± 4.58 mmHg, the mean difference was -0.8 mmHg, 95% CI [-5.11 to 3.51], p = 0.487) (Fig. 3).
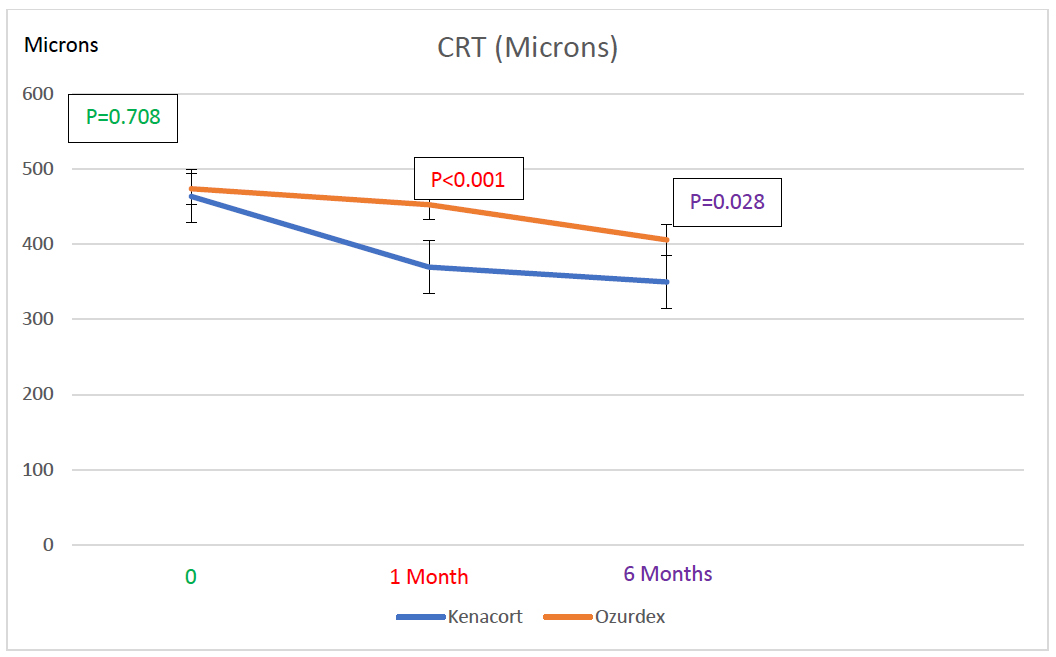
At the initial visit, CRT was 463.70 ± 106.59 µm in the ITA group and 473.70 ± 98.69 µm in the IDI group (p=0.708). At 1 month post-injection, CRT was 369.70 ± 75.83 µm in the ITA group and 452.60 ± 98.04 µm in the IDI group (p < 0.001). At the final follow-up visit, CRT was 349.8 ± 83.56 µm in the ITA group and 405.70 ± 106.91 µm in the IDI group (p= 0.028).
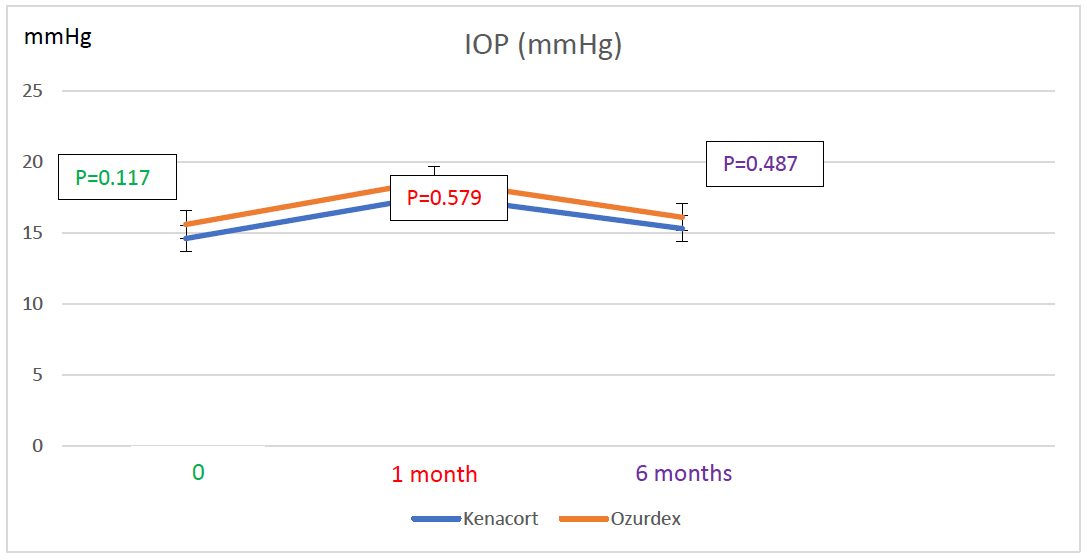
At the initial visit, IOP was 14.60 ± 1.22 mmHg in the ITA group and 15.60 ± 3.19 mmHg in the IDI group (p=0.117). At 1month post-injection, IOP was 17.60 ± 6.7 mmHg in the ITA group and 18.70 ± 8.46 mmHg in the IDI group (p=0.579). At the final follow-up visit, IOP was 15.30 ± 4.43 mmHg in the ITA group and 16.10 ± 4.42 mmHg in the IDI group (p= 0.487).
| - | ITA | IDI | p |
|---|---|---|---|
| BCVA at baseline | 0.89 ± 0.55 | 0.97 ± 0.52 | 0.556 |
| BCVA at 1 month | 0.57 ± 0.60 | 0.86 ± 0.55 | 0.054 |
| BCVA at 6 months | 0.58 ± 0.59 | 0.76 ± 0.68 | 0.287 |
| CRT at baseline | 463.70 ± 106.59 | 473.70 ± 98.69 | 0.708 |
| CRT at 1 month | 369.70 ± 75.83 | 452.60 ± 98.04 | <0.001 |
| CRT at 6 months | 349.80 ± 83.56 | 405.70 ± 106.91 | 0.028 |
| IOP at baseline | 14.60 ± 1.22 | 15.60 ± 3.19 | 0.117 |
| IOP at 1 month | 17.60 ± 6.70 | 18.70 ± 8.46 | 0.579 |
| IOP at 6 months | 15.30 ± 4.43 | 16.10 ± 4.42 | 0.487 |
3.4. Between-group Comparison
No significant difference was found between the two groups in terms of all variables, except for the CRT at 1 month and at 6 months, where a higher mean was seen in the IDI group compared to the ITA group (452.60 vs 369.70; p < 0.001 and 405.70 vs 349.80; p = 0.028, respectively) (Table 2).
3.5. Complications
The increase in IOP was treated with topical anti-glaucoma medications, with none requiring surgery. Cataract progression was treated surgically if the cataract reached grade 3 on the Lens Opacities Classification System (LOCS) III. None of the eyes reached grade 3 during the 6-month follow-up period.
4. DISCUSSION
4.1. Intravitreal Triamcinolone Acetonide (ITA)
In the ITA group, BCVA improved significantly from 0.89 ± 0.55 to 0.57 ± 0.60 logMAR at 1 month (p < 0.001) and remained stable at 6 months (0.58 ± 0.59, p = 1 vs. 1 month), while CRT decreased significantly from 463.70 ± 106.59 µm to 369.70 ± 75.83 µm at 1 month (p < 0.001) and further to 349.80 ± 83.56 µm at 6 months (p < 0.001 vs. baseline). These findings align with those reported in the SCORE study, which found that 1 mg and 4 mg of ITA, compared with observation, had similar visual acuity outcomes in the treatment of macular edema associated with CRVO over 1 to 2 years. It was found that the odds of reaching the primary outcome in the 1mg and 4mg groups were 5 times greater than in the observation group, with rates of 27%, 26%, and 7%, respectively. This is similar to our study, as a statistically significant improvement was found at 6 months in the ITA group compared with baseline.
Another study, conducted on 13 eyes, tested the efficacy of 4 mg ITA in the setting of macular edema associated with CRVO and showed that patients achieved improvement in BCVA and CRT. However, between 3 and 6 months, 4 of 13 enrolled eyes developed recurrent macular edema that was treated with a second injection, and 3 of the 4 eyes responded to the treatment [7]. This is consistent with our results, where 50% of patients required more than one injection, highlighting the often-transient nature of corticosteroid effects and the potential need for re-administration to maintain therapeutic benefit. Our study reinforces the role of ITA in achieving both anatomical and functional improvements in macular edema due to CRVO, while also emphasizing the importance of individualized retreatment strategies in the context of recurrent edema.
4.2. Intravitreal Dexamethasone Implant (IDI)
In the IDI group, BCVA improved significantly from 0.97 ± 0.52 to 0.86 ± 0.55 at 1 month (p < 0.001) and to 0.76 ± 0.68 at 6 months (p < 0.001 vs. baseline, p = 0.412 vs. 1 month), while CRT decreased significantly from 473.70 ± 98.69 µm to 452.60 ± 98.04 µm at 1 month (p < 0.001) and to 405.70 ± 106.91 µm at 6 months (p < 0.001 vs. both 1 month and baseline). Our results partially align with those of the GENEVA study, which evaluated the efficacy of dexamethasone implants in macular edema secondary to CRVO and BRVO compared to a sham group [8]. The study showed that patients who received the IDI had a significant improvement in BCVA and CRT at 90 days compared with the sham group. However, improvement at 6 months was statistically insignificant. This differs from our results, in which CRT continued to improve significantly at 6 months, suggesting a longer-lasting effect on retinal thickness. However, the gain in BCVA did not remain significant after the first month, as in the GENEVA study, which found that visual improvement tends to decrease over time.
Similarly, a clinical trial found that the effect of IDI does not last 6 months, and a retreatment protocol should be used [9]. Our findings partially support this pattern, as improvement in CRT was significant at 6 months, whereas that in BCVA was not. Moreover, a meta-analysis of 4 studies with a total of 99 patients evaluating the efficacy of switching from anti-VEGF to IDI for refractory macular edema in the setting of RVO reported that IDI improved BCVA and CRT, with efficacy lasting for 6 months [4].
Similarly, a study comparing IDI to anti-VEGF showed that IDI was more effective; however, its efficacy peaked at 2 months post-injection and then declined rapidly [10]. This is similar to our study, as at 1 month, patients showed significant improvement in CRT and BCVA. This improvement was maintained in CRT but not in BCVA at 6 months. Additionally, the transient improvement in BCVA observed in the IDI group at 1 month, followed by a non-significant change at 6 months, may be an indication of waning efficacy.
Our study demonstrated that IDI is effective in achieving a sustained reduction in CRT over a 6-month period. However, the absence of continued improvement in visual acuity beyond the first month may be attributed to the limited duration of action of the dexamethasone implant, emphasizing the potential need for timely retreatment in clinical practice.
4.3. ITA versus IDI
Between-group comparisons showed no significant differences in BCVA at baseline, 1 month, or 6 months, while CRT was similar at baseline but significantly lower in the ITA group at both 1 month and 6 months. These findings are consistent with those of another study that aimed at assessing the effectiveness and safety of ITA and IDI in treating macular edema associated with CRVO over a period of 6 months. No significant difference was found in terms of CRT and BCVA between IDI and ITA injections at any follow-up visit up to 6 months (5). In our study, eyes receiving IDI showed improvement at 1 month, which was not observed at 6 months; this suggests that IDI’s effect lasts for less than 6 months.
4.3.1. Complications
During the 6-month follow-up, cataract progression occurred in 40% of the ITA group and 20% of the IDI group, while IOP spikes were observed equally in both groups (40%), with all cases managed medically and no eyes requiring cataract surgery or surgical IOP intervention; additionally, IOP significantly increased at 1 month in the ITA group and then decreased at 6 months, while changes in the IDI group were not statistically significant, and no significant differences in IOP were found between groups at any timepoint.
The SCORE study shows that the 4 mg dose of ITA has a higher rate of complications (cataract progression and increased IOP) than the 1 mg dose, suggesting that the rate of complications might be dose-dependent [11]. While our study used the 4 mg dose of ITA, the observed complication rate was consistent with published data and manageable without surgical intervention.
In another study, 4 mg of ITA resulted in an increased IOP in 8 out of 13 eyes. This increase was easily controlled with topical treatment, as in our study. Cataract progression was noted in 5 out of the 7 phakic eyes in the study, and surgical extraction was required. The mean time to cataract progression was 14 months, suggesting that the 6-month duration of our study may have been insufficient to fully capture the extent of lens changes. This may explain why cataract progression, though more frequent in the ITA group, did not differ significantly between groups in our study.
As for 0.7 mg IDI, a study reported that over a 12-month observation period, 90 of 302 phakic eyes (29.8%) underwent cataract progression after 2 IDI implants, with only 4 eyes requiring surgery (1.3%) [12]. Similarly, another study reported that vision-impairing cataract was not observed in the one-time injection study group, whereas in the multiple injections group, 2 of 16 eyes underwent cataract extraction [13]. Moreover, 341 out of 1256 eyes had a ≥ 10-mmHg IOP increase from baseline (12.6% after the first treatment, and 15.4% after the second). An increase in IOP was usually transient and controlled with medication or observation [12]. Similar to our study, a meta-analysis of IDI use found no serious adverse effects [4]. Our findings are consistent with these reports, as no eyes in the IDI group required surgery, and cataract progression remained relatively limited.
One study reported that the relative risk of cataract progression was 3.5 times higher in the ITA group compared to the IDI group [5]. This contradicts our study, which found no statistical significance between the groups in terms of cataract progression, likely due to the small sample size.
Concerning previous anti-VEGF injections, patients who had received anti-VEGF treatment prior to the start of the study were not excluded. Although this represents a potential confounding factor, the comparison between both groups showed no significant differences (p=0.470), thereby reducing the risk of bias and supporting the validity of our results.
4.4. Clinical Implications
The findings of this study have valuable clinical implications for ophthalmologists worldwide, particularly those practicing in low-resource settings or serving underserved populations. It provides evidence-based guidance on cost-effective therapeutic strategies. ITA demonstrated comparable visual outcomes and even superior anatomical results compared to IDI, without increasing the risk of complications. Therefore, ophthalmologists may confidently consider ITA as a viable alternative in situations where access to IDI is limited by financial constraints. This is especially relevant in regions affected by economic hardship, humanitarian crises, or disruptions in the pharmaceutical supply chain.
4.5. Limitations
Our study has several limitations that should be acknowledged. It is a retrospective, non-randomized design, with a relatively small sample size (n=60 eyes, 30 per group), which may limit statistical power, particularly for detecting less frequent complications. The follow-up duration was limited to 6 months, which may not be sufficient to evaluate long-term outcomes, such as recurrence rates, cataract progression, and sustained efficacy. Furthermore, potential confounding factors may have influenced the results, including prior anti-VEGF treatment in 70% of patients and differences in treatment frequency between groups, both of which should be taken into account in later studies. A selection bias is also possible, as patients were recruited from a single hospital.
CONCLUSION
In conclusion, at 1 and 6 months, our findings reported no statistically significant difference between the two groups in best-corrected visual acuity (BCVA), central retinal thickness (CRT), or complications, except at the 1-month mark, when ITA was superior to IDI in CRT improvement. Consequently, ITA could be considered a reliable and effective alternative to IDI while maintaining safety.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: P.R.: Study conception and design; J.N.: Data curation; M.R.S.: Investigation; A.J. and A.S.: Methodology; A.S.: Validation; C.A.D.: Writing, reviewing, and editing; E.J.: Writing the original draft preparation. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| ITA | = Intravitreal Triamcinolone Acetonide |
| IDI | = Intravitreal Dexamethasone Implant |
| CRVO | = Central Retinal Vein Occlusion |
| BCVA | = Best Corrected Visual Acuity |
| Me | = Macular edema |
| LOCS | = Lens Opacities Classification System |
| CRT | = Central Retinal Thickness |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Eye and Ear Ethics Committee provided ethical approval for the research done. The study is retrospective one so no clinical trial was done.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
The patients provided written informed consent, explicitly granting approval for the utilization of their data within the study.
AVAILABILITY OF DATA AND MATERIALS
The source of data and material is from Eye and Ear Hospital International database and can be obtained by the corresponding author [C.A.D] on reasonable request.
ACKNOWLEDGEMENTS
The authors would like to thank all patients who contributed to the study.


