All published articles of this journal are available on ScienceDirect.
Primary Anterior Conjunctival Cyst Developed Over an Encapsulated Ahmed® Glaucoma Valve Device: A Case Report
Abstract
Background
Several studies have recommended glaucoma drainage devices (GDDs), particularly the use of the Ahmed glaucoma valve, as the primary surgical procedure. Nevertheless, surgical hypotony, capsular fibrosis, and tube failure are among the side effects of these devices. In this study, we report a case of the development of a conjunctival cyst as a complication of the surgical implantation of the Ahmed glaucoma valve device.
Case Presentation
A 21-year-old man who was diagnosed with bilateral congenital glaucoma presented with a persistently high intraocular pressure even after undergoing bilateral trabeculectomy and trabeculotomy with mitomycin C. After that, Ahmed glaucoma valve was employed, which led to an improvement in IOP postoperatively; however, upon follow-up, we noticed by clinical examination the development of a large encapsulated subconjunctival cyst, and then prompt surgical excision of the cyst was performed.
Conclusion
Formation of a large conjunctival cyst is one of the complications that can be noticed post-AGV (Ahmed® glaucoma valve) use. This case showed the surgical resection of the cyst to be essential and effective. More investigations are needed to address this complication with proper preoperative counselling for any postoperative GGD complications occurring in the patients.
1. INTRODUCTION
Glaucoma drainage devices have become a more favorable option for the management of refractory glaucoma, which is a leading cause of irreversible blindness [1]. The Ahmed® glaucoma valve (AGV) device was approved by the Food and Drug Administration in 1993. The device was manufactured and designed to immediately reduce and control intraocular pressure (IOP) with its unique flow restrictor mechanism that avoids excessive drainage. Further, it was particularly designed for refractory, neovascular, pediatric, and complicated glaucoma cases associated with a higher risk of failure with subsequent persistence of high IOP levels, which are observed in patients who have undergone conventional filtration surgeries, such as trabeculectomy [2].
Due to their safety and efficacy, the use of glaucoma drainage devices has increased significantly in recent years. These devices, particularly the AGV, are effective in reducing the risk of associated early and late postoperative complications, such as hypotony, capsular fibrosis, tube erosion, cataract formation, diplopia, cystoid macular edema, and other rare complications, such as scleral perforation and expulsion or migration of the plate. However, they cannot completely inhibit them [3, 4].
Conjunctival cysts, also known as Tenon cysts, are benign lesions that are either congenital or acquired in origin, and they usually develop after surgery or trauma [5]. The development of encapsulated cysts was observed after the formation of an impermeable bubble to the aqueous humor that adhered to the episcleral tissue, followed by encapsulation and an increase in IOP, in approximately 23% of AGV cases [1]. Herein, we have presented a case of a primary anterior conjunctival cyst over an encapsulated AGV implant and the management procedure adopted.
2. CASE PRESENTATION
2.1. Chief Complaint
The patient complained of a gradually increasing swelling in the superotemporal aspect of the conjunctiva of the left eye that was associated with foreign body sensation. The anterior and posterior segments were examined. Slit-lamp photography and ultrasound biomicroscopy of the eye were performed to visualize the structures and anterior segment of the eye.
2.2. Patient Details
A 21-year-old male patient from Al Qassim, which is a province of Saudi Arabia, first presented to the emergency department of King Khaled Eye Specialist Hospital in Riyadh, Saudi Arabia, as an infant (45 days of age). The patient, back then, presented with redness and a cloudy cornea in the right eye for 2 weeks. Initial examination revealed visual acuity in both eyes, with IOP 37 mmHg in the right eye and 30 mmHg in the left eye.
Biomicroscopic slit-lamp examination showed mild conjunctival congestion, an enlarged horizontal corneal diameter (12 mm), mild corneal edema, a deep, quiet anterior chamber, a normal crystalline lens, and a round and reactive pupil. The following were observed in the left eye: a quiet conjunctiva, clear cornea with a horizontal corneal diameter of 10.5 mm, deep quiet anterior chamber, normal crystalline lens, and round and reactive pupil. The fundus examination results were as follows: a 0.4 and 0.5 optic nerve cup-to-disc ratio on the right and left eye, respectively.
Based on these findings, the patient was diagnosed with bilateral congenital glaucoma, and examination under anesthesia was performed for potential surgical intervention due to an uncontrolled IOP.
During examination under anesthesia on November 15, 2003, the initial attempt at goniotomy was unsuccessful due to poor visibility of the anterior chamber structures caused by corneal haze. Consequently, bilateral trabeculectomy and trabeculotomy with mitomycin C were performed. Despite these interventions, the IOP of the left eye remained elevated during the follow-up visits, with measurements consistently exceeding 25 mmHg. To address the persistently elevated IOP, trabeculectomy with mitomycin C (0.2 mg/mL for 3.5 min) was conducted on the left eye in April 2008. The patient’s IOP initially reduced. However, it gradually increased again over the subsequent months, reaching levels >24 mmHg on maximal topical IOP-lowering medical therapy.
In July 2011, Ahmed® valve implantation with a pericardial patch graft was performed in the left eye to provide an alternative outflow pathway for aqueous humor. Postoperatively, the IOP initially stabilized at approximately 18 mmHg. However, it began to increase over time, which required further follow-up and management. By September 2011, a large encapsulated superotemporal subconjunctival cyst was noted in the left eye during the follow-up visits. Ultrasound biomicroscopy was not successful in detecting the cyst. Nevertheless, clinical examination confirmed its presence (Fig. 1), which prompted the surgical excision of the cyst in October 2011.
2.3. Description of the Procedure
After routine sterilization with Betadine 5% and surgical draping, a wire lid speculum was applied to the left eye. A traction suture with #7-0 Vicryl was placed at the 12 o’clock position, and the globe was rotated inferiorly for better visualization and exposure. A large cyst extending from the posterior conjunctiva to the limbal area was identified. Dissection began at the dome of the cyst, approximately 9 mm from the limbus. Then, subconjunctival dissection proceeded via the Tenon layer. Prolapsed fat and suspected lacrimal gland tissue were noted superotemporally. Complete excision of the conjunctival cyst was achieved inferiorly, temporally, and nasally, with partial superior excision where the conjunctival cyst was attached to the posterior AGV plate encapsulation. Aspiration of the posterior encapsulated cyst released aqueous fluid, thereby partially deflating the anterior conjunctival cyst. Viscoelastic was injected, and excision was extended to the edge of the AGV plate.
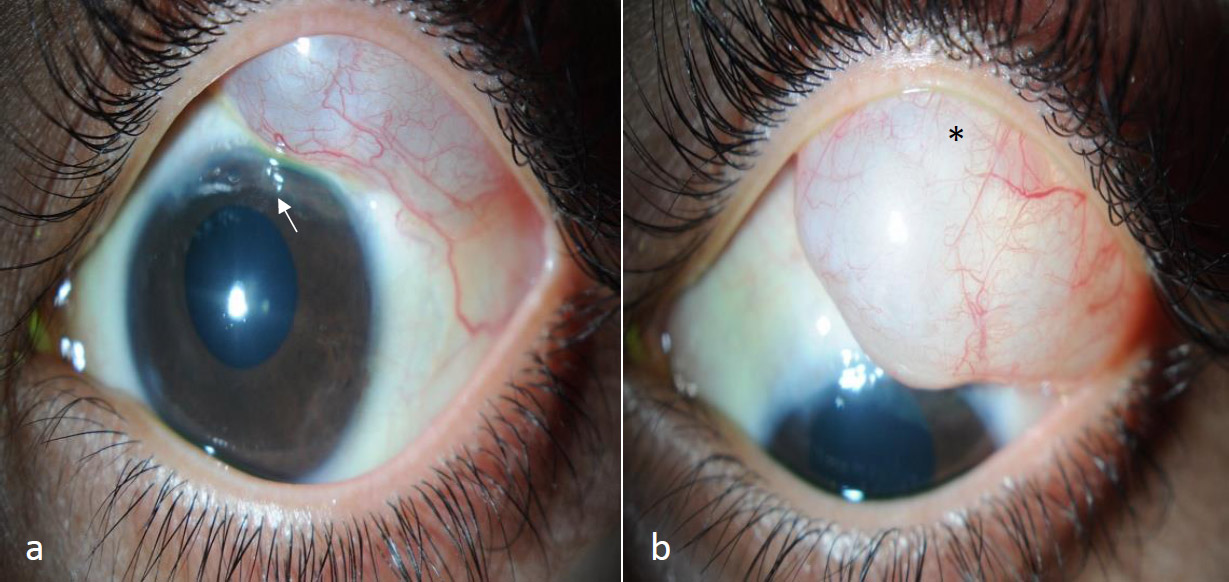
The slit-lamp image shows a large, well-defined subconjunctival cyst located in the superotemporal quadrant of the left eye and extending up to the limbus, as observed in the primary gaze (a) and the down gaze (b) with the AGV tube tip visible in the anterior chamber (a: white arrow) and faintly visible under the AGV plate edge as observed through the cyst (b: asterisk).
Thereafter, the posterior capsule over the plate was also resected. Next, the Tutoplast graft was placed over the tube and secured with four #10-0 Nylon sutures at each corner. The Tenon layer was sutured using #7-0 Vicryl, and the conjunctiva was closed with watertight #9-0 Vicryl sutures. A small buttonhole identified 3 mm from the limbus was repaired with a single #9-0 Vicryl suture, and viscoelastic was replaced with a balanced salt solution. Then, fluorescein testing confirmed the absence of leaks. Finally, cefazolin (50 mg) and betamethasone (3 mg) were administered subconjunctivally, and the eye was dressed with Maxitrol ointment and atropine ointment.
Two surgical specimens, one from the subconjunctival cyst and one from the posterior AGV plate capsule, were sent for histopathological analysis.
2.4. Specimen Examination Results
The microscopic and histopathological analysis results were as follows: a subconjunctival cyst measuring 10 × 4 mm2, containing an irregular cystic lumen lined by endothelial-like cells with adjacent fibroblasts, smooth muscle fibers, and dilated blood vessels, was found within the cyst wall without an identifiable epithelial lining. These findings suggested a dilated lymphatic channel, likely secondary to the surgical intervention and AGV implantation. The posterior AGV plate capsule, measuring 10 × 2 mm2, contained dense, irregular connective tissues with numerous blood vessels and adjacent loose fibrovascular connective tissues. However, these results were consistent with fibrosis related to the AGV implant.
The excision of the subconjunctival cyst and posterior plate capsule was effective in improving IOP control. Postoperatively, the patient’s IOP stabilized at 13 mmHg in both eyes on full topical IOP-lowering medications. In the last follow-up visit on September 11, 2023, ultrasound biomicroscopy did not show a cyst, and the IOP values were 16 and 17 mmHg, respectively.
3. DISCUSSION
Glaucoma can be effectively managed with a glaucoma drainage device, such as the AGV implant, which lowers IOP to a satisfactory level. However, several postoperative complications, such as hypotony in the early and late stages after surgery, severe capsule fibrosis surrounding the plate, erosion of the tube or plate border, and, rarely, infection, have been reported [6]. The incidence rate of encapsulated cyst formation is 5%–10% [7]. In a previous study, an encapsulated plate was a late postoperative complication in 51 of 124 eyes in 99 (41%) patients [8]. Al Atiah et al. showed that a large subconjunctival cyst still developed even after AGV revision and removal of encapsulation in a few cases [5].
In this case, a postoperative subconjunctival cyst was found over an encapsulated AGV plate, and bilateral trabeculectomy and trabeculotomy with mitomycin C were not effective in decreasing IOP. Therefore, additional AGV implantation is required for subsequent IOP elevation and the formation of a subconjunctival cyst over an encapsulated AGV plate. This case showed that the surgical removal of the subconjunctival cyst could effectively lower IOP. We hypothesized that the main cause of failed IOP reduction in the left eye in this case was due to both the development of a subconjunctival cyst and the excessive capsular fibrosis surrounding the plate, preventing the diffusion of aqueous humor [9]. Nevertheless, a subconjunctival cyst may have developed due to the implantation of conjunctival tissues or dragging of the conjunctiva during implantation [10]. The patient underwent excision of the subconjunctival cyst with complete resolution. The IOP was reduced, and no further recurrences were observed.
CONCLUSION
Postoperatively, a large conjunctival cyst can develop over an encapsulated AGV, which is considered a rare complication. This is one of the limited case reports showing the occurrence of this complication. The IOP was satisfactory after complete excision of the cyst without recurrence during revision. Thus, in addition to preoperative counseling, more assessments should be conducted postoperatively to address this complication.
AUTHORS’ CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| GDDs | = Glaucoma drainage devices |
| AGV | Ahmed® glaucoma valve |
| IOP | = Intraocular pressure |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by King Khalid Eye Specialist Hospital, Riyadh, Saudi Arabia, with approval number RD/26001/IRB/0025-24.
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the individual for the publication of the details of the medical case and any accompanying images.
DATA AVAILABILITY
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
ACKNOWLEDGEMENTS
The authors thank the patient for graciously consenting to participate in this research study.


