All published articles of this journal are available on ScienceDirect.
Recent Advances and Future Directions of Artificial Intelligence in Glaucoma Management
Abstract
Glaucoma, a leading cause of irreversible blindness worldwide, presents substantial challenges in clinical diagnosis and long-term management due to its often insidious early progression and the irreversible nature of late-stage optic nerve damage. However, rapid advancements in artificial intelligence technologies, particularly in machine learning, deep learning, and large language models, are transforming ophthalmic practice. AI is now being extensively applied across the spectrum of glaucoma care, from screening and precise diagnosis to optimizing treatment and supporting long-term patient management. This review systematically examines the latest applications of AI in glaucoma, highlighting multidimensional innovations. These applications span a wide range, including sophisticated image analysis, the identification of novel molecular biomarkers, prediction of treatment response, and advanced surgical planning. The paper also discusses key challenges and future development directions of these technologies, aiming to provide new insights for glaucoma management.
1. INTRODUCTION
Glaucoma, characterized by distinctive optic nerve atrophy and visual field defects, often presents asymptomatically in its early stages, leading to severe visual impairment as it progresses [1, 2]. Currently, reducing intraocular pressure (IOP) remains the most definitive and controllable treatment strategy [3, 4]. However, clinical practice has shown that disease progression can still occur even at target IOP levels, necessitating novel therapeutic approaches beyond IOP control [5]. Further complicating glaucoma management are the subjectivity of diagnosis, the resource-intensive nature of screening methods, and challenges with long-term patient adherence to treatment [6-8]. These inherent complexities underscore the critical need for advanced tools to improve early detection, diagnosis, and personalized management.
In recent years, rapid advancements in artificial intelligence (AI) have offered new perspectives for glaucoma management [9, 10]. AI can be understood as the ability of computers or robots to reproduce human intelligence through software and algorithms. Machine learning (ML), a subfield of AI, learns patterns from data for applications such as medical image analysis [11, 12]. Deep learning (DL), another important branch of AI, uses multilayered neural networks to automatically extract hierarchical features and identify complex information from large datasets, such as medical images or extensive clinical records [13, 14]. Large language models (LLMs) process human language, excelling at extracting clinical information from unstructured text such as electronic health records (EHRs) and supporting multimodal data analysis (e.g., combining imaging and text information) [15, 16]. Additionally, LLMs can simplify patient education through natural language interaction, enhance professional literacy, and assist clinicians with literature retrieval and treatment planning [17, 18].
The integration of these AI technologies is poised to improve diagnostic accuracy, optimize treatment decisions, predict disease progression, and enhance patient engagement and adherence. This review details the applications of these AI technologies in glaucoma management, highlighting their potential to transform care from an experience-driven approach to data-driven precision medicine, thereby offering new avenues for early diagnosis, treatment, and individualized intervention.
2. APPLICATIONS OF AI IN GLAUCOMA MANAGEMENT
Ophthalmology offers significant advantages for AI applications (Table 1). It relies heavily on various imaging technologies, such as fundus photography and optical coherence tomography (OCT), which AI can analyze in depth to assist clinicians in the early screening and diagnosis of glaucoma [19, 20]. The inherent data-rich environment of ophthalmology, characterized by the extensive use of high-resolution imaging, provides a fertile foundation for the development and application of AI algorithms. This fundamental advantage is a primary reason why AI has achieved such widespread and impactful integration within this specialty, setting the stage for its diverse applications in glaucoma management (Fig. 1) [21].
| Application Area | Data Type | AI Model | Specific Role | Related Research |
|---|---|---|---|---|
|
Early Screening and Detection of Glaucoma |
Fundus Photograph y | CNNs | Detection of early GON | Liu et al. [22] |
| DL | Predict referable GON | Phene et al. [23] | ||
| CNNs | Predict the risk of glaucoma development. | Thakur et al. [27] | ||
| ViTs | Classification of glaucoma | Tohye et al. [26] | ||
| CNNs | Discover new glaucoma-related genetic loci | Han et al. [29], Alipanahi et al. [30] | ||
| OCT | ChatGPT-4 | Detection of glaucoma | AlRyalat et al. [32] | |
| PointNet | Detection of glaucoma | Thiéry et al. [24] | ||
| 3D CNNs | Detection of glaucoma | Noury et al [25] | ||
| Molecular Genetic Data | ML | Identify key candidate genes. | Dai et al. [28] | |
| Clinical Cases | ChatGPT | Detection of glaucoma | Delsoz et al. [31] | |
| Glaucoma Diagnosis | Cup-to-disc ratio | retinIA | Diagnosis of suspected glaucoma | Camacho et al. [33] |
| Predicting Glaucoma Progression | OCT | LSTM | Predict glaucoma progression | Mandal et al. [38] |
| GTNs | Identify visual field progression. | Hou et al. [39] | ||
| - | Siamese Neural Network | Identify rapid visual field progression | Mohammadzadeh et al. [40] | |
| Visual Field | AI-driven dashboard | Predict glaucoma progression | Yousefi et al. [41] | |
| clinical data | Convolutiona l LSTM Network |
Predict glaucoma progression | Dixit et al. [42] | |
| EHRs | ChatGPT-4 | Accuracy in predicting conversion of ocular hypertension to glaucoma | Huang et al. [43] | |
| Guiding Glaucoma Treatment | EHRs | Random Forest Model | Predict trabeculectomy outcomes | Banna et al. [46] |
| Predict glaucoma surgery failure. | Barry et al. [47] | |||
| Anterior Segment OCT | Classification Tree | Predict filtering surgery outcomes. | Agnifili et al. [48] | |
| Slit Lamp Images | Residual Network | Differentiate the filtration bleb function after trabeculectomy | Mastropasqu a et al. [49] | |
| Clinical Cases | ChatGTP | Predict optimal surgical approach | Carlà et al. [52] | |
| Molecular Databases | LLMs | Identify potential therapeutic targets and search for possible drug |
Tu et al. [56] | |
| - | - | - | candidates | - |
|
Glaucoma Chronic Disease Managemen t |
EHRs and Imaging | LLMs | Serve as a glaucoma “health advisor | Bahir et al. [62] |
| Self-reported Data | XGBoost | Assess high-risk glaucoma patients | Ravindranath et al. [66] | |
| EHRs | CNNs | Identify high-risk glaucoma patients | Ravindranath et al. [67] |
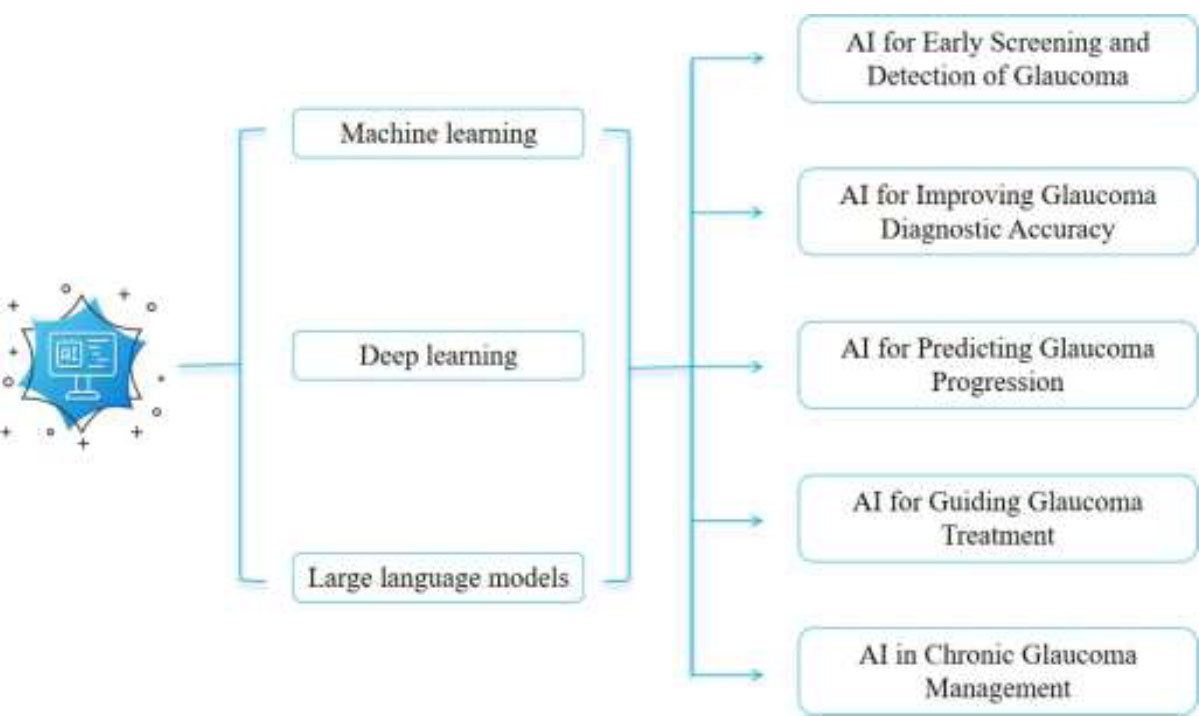
Applications of AI in glaucoma management.
2.1. AI for Early Screening and Detection of Glaucoma
Early screening and detection are cornerstones of glaucoma management, and AI plays a crucial role in this process, primarily through image analysis and molecular genetic data mining. In fundus photography–based detection, DL techniques, especially convolutional neural networks (CNNs), effectively identify glaucomatous optic neuropathy (GON) from fundus images. Liu et al. reported that CNNs achieved an AUC of 0.996 in detecting GON, demonstrating high sensitivity and specificity [22]. Similarly, Phene et al. utilized a DL model to analyze fundus photographs from primary care settings and predict referable GON patients with an AUC of 0.945, exhibiting higher sensitivity and comparable specificity to glaucoma specialists [23].
AI is also increasingly applied to OCT analysis. DL models can effectively identify glaucoma by analyzing retinal nerve fiber layer (RNFL) thickness, optic disc structure, and OCT angiography data. Thiéry et al. showed that DL models such as PointNet achieved an AUC of 0.95 for glaucoma diagnosis based on optic nerve OCT images, indicating high accuracy [24]. Noury et al. used 3D CNNs to analyze optic nerve OCT from different datasets and consistently demonstrated high accuracy in glaucoma identification [25]. In addition, emerging architectures like Vision Transformers (ViTs) show promising applications in glaucoma management. Tohye et al. reported an accuracy of 93%, significantly outperforming conventional methods [26].
These AI systems exhibit a remarkable capacity to identify subtle structural changes that are often imperceptible to the human eye. Beyond detection, they also help predict the risk of glaucoma development, enabling truly early identification. As shown in Thakur’s research, their DL model achieved an AUC of 0.77 in predicting glaucoma development 4 to 7 years before onset and an AUC of 0.88 in predicting development 1 to 3 years prior to onset [27].
AI further demonstrates immense potential in molecular genetic data analysis. ML algorithms have successfully analyzed RNA sequencing data, identifying key candidate genes associated with glaucoma, such as ENO2, NAMPT, and ADH1C [28]. Furthermore, researchers have advanced large-scale genome-wide association studies through DL-assisted phenotyping of fundus images (e.g., quantifying the vertical cup-to-disc ratio). As shown in research by Han [29] and Alipanahi et al [30], AI models aided in discovering multiple new genetic loci related to optic disc morphology and glaucoma susceptibility, providing crucial insights for unraveling disease pathogenesis and identifying novel biomarkers.
LLMs are also increasingly applied in glaucoma detection. They can analyze text information from EHRs and, when combined with image recognition systems, can assist in interpreting fundus photographs, visual field tests, and OCT images for early identification of glaucoma. One study reported that ChatGPT achieved 72.7% accuracy in diagnosing glaucoma from detailed clinical cases, occasionally outperforming senior ophthalmology residents [31]. Another study based on ChatGPT-4 achieved a diagnostic accuracy of 90% [32].
The application of AI in glaucoma management is shifting the model from “passive diagnosis” to “active warning” by identifying high-risk individuals and predicting disease risk, thereby establishing a strong foundation for early intervention.
2.2. AI for Improving Glaucoma Diagnostic Accuracy
Enhancing diagnostic accuracy is a core component of glaucoma management, and AI models play a significant role in improving both the precision and efficiency of glaucoma diagnosis. A comparative study demonstrated that the AI tool retinIA outperformed ophthalmology residents in diagnosing suspected glaucoma and in cup-to-disc ratio estimation [33].
Crucially, the study also emphasized that a synergistic approach combining AI assessment with clinician judgment yields higher sensitivity in glaucoma diagnosis compared to either method alone. This finding highlights a central role of AI in medicine [34]: it serves as a powerful assistive tool, providing objective, consistent, and accurate analyses to augment—rather than replace—human expertise. This is particularly beneficial for clinicians with less experience or those working in high-intensity environments [35].
Furthermore, the continuous optimization and increasing accessibility of AI offer new opportunities for clinicians in resource-limited primary care settings, enabling them to diagnose glaucoma more efficiently and accurately [36]. This ability of AI to bridge knowledge and resource gaps effectively democratizes access to high-level diagnostic capabilities that might otherwise be concentrated in specialized centers, thereby improving patient outcomes in underserved areas.
2.3. AI for Predicting Glaucoma Progression
Predicting disease progression is a critical aspect of glaucoma management. In recent years, AI technologies, including complex architectures such as Recurrent Neural Networks (RNNs) and transformer models, have excelled at analyzing longitudinal data from OCT and visual field examinations, enabling relatively accurate predictions of disease progression and trajectory [37].
Long Short-Term Memory (LSTM) networks, a type of RNN, can differentiate whether RNFL thinning observed on OCT is due to glaucoma progression or age-related changes. Mandal et al. [38] reported that this model achieved a diagnostic efficacy of AUC 0.498 in identifying such changes. Gated Transformer Networks (GTNs) demonstrate significant advantages in predicting visual field damage based on OCT; a study by Hou et al. showed an AUC of 0.97 [39]. Siamese Neural Networks, primarily used for comparing paired data, can predict visual field progression by analyzing optic disc images and baseline RNFL thickness. In a study by Mohammadzadeh et al., this AI model achieved an AUC of 0.911 in identifying rapid visual field progression [40].
To further enhance clinical utility, AI-driven dashboards are being developed to provide physicians with intuitive visual representations of visual field progression. Research by Yousefi et al. supports their high sensitivity and specificity in predicting disease progression [41]. Additionally, AI models that combine clinical data (such as cup-to-disc ratio, central corneal thickness, and intraocular pressure) with visual field information have shown strong predictive accuracy. In a study by Dixit, their predictive performance reached an AUC of 0.89–0.93 [42].
LLMs uniquely process patient text information, simulating the thought process of experienced clinicians by integrating imaging, functional assessments, and medical symptoms to help identify potential glaucoma risks [43]. Combining LLM-driven text analysis with DL-driven image analysis enables the development of multimodal AI models, contributing to a more comprehensive and detailed diagnostic and prognostic evaluation system [37].
The application of these AI models in predicting glaucoma progression will further drive the transformation of glaucoma management from a “reactive treatment” approach to a “proactive intervention” model, laying a solid foundation for optimizing long-term patient management strategies and improving quality-of-life outcomes.
2.4. AI for Guiding Glaucoma Treatment
Beyond screening, detection, diagnosis, and prediction, AI is widely applied in glaucoma treatment. It guides treatment decisions, predicts treatment responses, accelerates drug discovery, and even improves surgical precision, thereby advancing glaucoma management towards more personalized and efficient directions.
2.4.1. AI for Predicting Treatment Response
Determining the most suitable treatment plan for each patient is a key aspect of glaucoma management [44]. Currently, many AI models under development can predict glaucoma treatment responses by analyzing diverse data sources (e.g., EHRs, OCT, visual fields, and IOP) [45]. Several ML models are also used to predict outcomes of glaucoma filtering surgery. For instance, Banna et al. [46] found that random forest models achieved an AUC of 0.74 in predicting trabeculectomy outcomes, while Barry et al. [47] demonstrated their superior performance in predicting glaucoma surgery failure.
Additionally, AI can support surgical prognosis by analyzing specific anatomical features. For example, Agnifili et al [48]. predicted filtering surgery outcomes by classifying conjunctival stromal thickness measured by anterior segment OCT using decision tree analysis, achieving an AUC of 0.784. Mastropasqua et al [[49] assessed the function of filtration blebs after trabeculectomy using ResNet to analyze slit-lamp examination images, achieving an accuracy of 74%. These advancements indicate that AI can support prognostic assessment in glaucoma surgery, helping optimize treatment choices and facilitate the development of personalized glaucoma treatment plans.
2.4.2. AI for Guiding Treatment Decisions
LLMs are capable of processing complex text and identifying key patient information from EHRs, thus gradually becoming important auxiliary tools in developing treatment plans and clinical decisions [50, 51]. For example, ChatGPT predicts the optimal surgical approach for patients by analyzing clinical cases, achieving an accuracy of 78% and often demonstrating predictive ability comparable to glaucoma experts [52]. Furthermore, LLMs can serve as dynamic knowledge bases, providing the latest medical evidence to support evidence-based medicine decisions, helping doctors stay updated with rapidly evolving treatment perspectives. As “clinical co-pilots,” these AI models can integrate vast amounts of patient clinical data with the latest medical knowledge to assist clinicians in making complex glaucoma treatment decisions [53, 54].
2.4.3. AI for Developing New Therapies
AI also holds promising prospects in new drug development, capable of discovering novel drugs for glaucoma [55]. AI models such as LLMs and graph neural networks can rapidly analyze vast molecular databases, predict intermolecular interactions, identify potential therapeutic targets, and search for possible drug candidates. A typical example involves AI screening compounds that target receptor-interacting protein kinase 3 (RIPK3), an enzyme that mediates necroptosis of retinal ganglion cells (RGCs). With AI, Researchers identified HG9-91-01 as a promising drug candidate, which showed protective effects on RGCs in preclinical studies [56]. This undoubtedly shortens drug screening time and reduces costs, which is particularly crucial for finding new neuroprotective therapies related to glaucoma.
2.4.4. AI for Optimizing Surgical Procedures
Currently, the application of AI in ophthalmic surgery is still in its early stages and is primarily focused on optimizing surgical procedures through robotic-assisted technology [57, 58]. Robotic platforms specifically designed for ophthalmic microsurgery, such as the “Steady Hand” system and the “Intraocular Robotic Interventional Surgical System (IRISS),” can minimize hand tremors and enable more precise manipulations, thereby improving surgical safety and effectiveness [59, 60]. The use of these systems supports the broader adoption of minimally invasive glaucoma surgeries (MIGS), which require a high degree of dexterity. Integrating AI with these robotic platforms is expected to further refine surgical techniques and optimize surgical outcomes.
Additionally, AI can serve as an important auxiliary tool during surgery. Ahuja et al. [61] demonstrated that integrating AI with imaging tools such as fundus photography and OCT provides real-time, high-resolution image analysis and guidance for surgeons, greatly enhancing surgical safety.
2.5. AI in Chronic Glaucoma Management
In recent years, AI systems such as LLMs and chatbots have increasingly assumed the role of “health advisors” for glaucoma patients [51, 54, 62]. They simplify complex medical literature, extract key information, and make intricate medical knowledge more accessible to patients with varying levels of health literacy. Furthermore, platforms like ChatGPT, Gemini, and Bing AI provide patients with basic glaucoma information, explanations of medical terminology, and overviews of potential treatment options through interactive Q&A sessions. They can also generate personalized educational materials and tailored medical consultations. The use of these AI tools enhances the efficiency of doctor-patient communication and improves patient adherence, enabling patients to play a more active role in their own care.
However, caution is warranted with these “health advisors,” as research indicates notable variability in their accuracy, completeness, and readability [63]. Studies suggest that patient education materials from professional organizations such as the American Academy of Ophthalmology (AAO) exhibit higher accuracy and readability compared to responses generated by AI tools like ChatGPT [64, 65].
AI models also play an important role in glaucoma risk assessment by assisting in the identification of high-risk populations. The XGBoost model [66] achieved an AUC of 0.890 in assessing glaucoma risk based on self-reported data, while a one-dimensional CNN model [67] achieved an AUC of 0.863 in accurately identifying glaucoma patients using EHRs, demonstrating strong predictive performance.
The introduction of AI into glaucoma patient management is reshaping the traditional two-party doctor-patient relationship into a more complex three-party relationship involving doctors, patients, and AI. This shift requires clinicians to develop new communication strategies to help patients understand and appropriately integrate AI-generated information, ensuring that AI enhances the therapeutic relationship rather than complicating it.
3. CHALLENGES IN AI APPLICATION
While the application of AI in glaucoma management holds vast promise, it also faces several challenges, including issues related to data quality, model transparency, interpretability, and ethical considerations. Actively and comprehensively addressing these challenges is essential to fully leverage the potential of these technologies.
Developing robust AI models requires large quantities of high-quality, diverse datasets, yet limited data and poor data quality remain significant obstacles [68, 69]. For instance, in prospective hospital studies with limited glaucoma subjects, small datasets can lead to “overfitting,” wherein models over-adapt to training data and perform inaccurately on new data. Additionally, variations across populations, imaging equipment, and acquisition protocols increase data heterogeneity, meaning that AI models trained on data from a specific institution or population often struggle to maintain consistent performance in different environments. Therefore, rigorous clinical validation using diverse, multi-center datasets is imperative to enhance model generalizability.
Moreover, many advanced AI models, particularly complex DL models, are difficult for clinicians to fully understand and trust due to their “black box” nature [70]. The development of explainable AI (XAI) techniques, such as Shapley Additive Explanations (SHAP), aims to improve model transparency and interpretability, but their practical applicability and effectiveness in clinical settings still require further validation [71]. Additionally, LLMs may exhibit “hallucinations,” generating information that appears plausible but is incorrect, which poses significant risks in clinical applications [72, 73]. Therefore, providing accurate training data and rigorously validating AI-generated information are essential to prevent the dissemination of wrong or harmful advice.
With respect to ethics, AI tools may heighten the risk of exposing sensitive patient information, making strict adherence to data privacy regulations crucial during their use [74-76]. Accountability is also a key concern, requiring clear delineation of responsibility when AI systems contribute to patient harm: Is it the AI vendor who built the algorithm, the clinician who used the tool and acted on its output, or the healthcare institution that deployed the AI? Establishing clear accountability mechanisms is essential for building trust and ensuring patient safety [77]. Furthermore, ensuring that AI tools perform fairly across diverse populations is critical for promoting equitable access to AI-driven healthcare innovations and reducing existing health disparities.
Given the current limitations of artificial intelligence—including potential biases, the “black box” nature of certain models, and the possibility of errors such as hallucinations in large language models—maintaining a “human-in-the-loop” approach is not merely a transitional phase but an ethical and practical necessity. In this model, clinicians oversee AI-generated outputs, evaluate recommendations, and ultimately make clinical decisions. This oversight serves as a crucial safeguard against risks associated with AI errors, thereby fostering responsible innovation and strengthening trust in emerging AI technologies.
CONCLUSION
AI is profoundly reshaping glaucoma management. Its exceptional performance in image recognition, disease prediction, and personalized intervention provides powerful support for clinical decision-making, patient education, and long-term management, while also opening new avenues for novel drug discovery and treatment optimization. The application of AI models is poised to transform glaucoma management from traditional “passive diagnosis” to “active early warning,” fostering a more predictive, precise, and accessible diagnostic and therapeutic paradigm.
Future development will focus on building multimodal AI systems by integrating diverse data—including imaging, functional assessments, genomics, EHRs, and patient self-reports—to achieve multi-dimensional modeling and dynamic tracking of individual patient conditions.
As AI advances, the role of ophthalmologists will also evolve—from traditional independent clinicians to AI-assisted comprehensive decision-makers. Future clinical practice will emphasize physicians’ critical interpretation of AI outputs, integration of complex clinical scenarios, and humanistic care in doctor-patient communication. This “human–machine collaboration” model will enhance the safety and effectiveness of diagnosis and treatment while enriching the medical profession with new dimensions.
In conclusion, the integration of AI is ushering in a new era of glaucoma management. Only by consistently advancing technological innovation with a rigorous scientific attitude, prudent ethical principles, and a clinically oriented perspective can AI be truly transformed into a powerful driving force for improving the entire continuum of glaucoma care. Future glaucoma management will be data-driven, patient-centered, and technology-supported, steadily progressing toward more precise, efficient, and personalized approaches.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: W.T.: Collected the literature, prepared the tables and figures, and drafted the preliminary version of the review article; Y.H. and J.Z.: Revised the manuscript; and Y.D.: Further revised the text and submitted the final article. All authors have read and agreed to the published version of the manuscript.
LIST OF ABBREVIATIONS
| IOP | = Intraocular pressure |
| AI | = Artificial intelligence |
| ML | = Machine learning |
| DL | = Deep learning |
| LLMs | = Large language models |
| EHRs | = Electronic health records |
| OCT | = Optical coherence tomography |
| CNNs | = Convolutional neural networks |
| GON | = Glaucomatous optic neuropathy |
| RNFL | = Retinal nerve fiber layer |
| ViTs | = Vision Transformers |
| RNNs | = Recurrent neural networks |
| GTNs | = Gated transformer networks |
| RIPK3 | = Receptor-interacting protein kinase 3 |
| RGCs | = RGCs = Retinal ganglion cells |
| IRISS | = Intraocular Robotic Interventional Surgical System |
| MIGS | = Minimally invasive glaucoma surgeries |
| AAO | = American Academy of Ophthalmology |
| SHAP | = Shapley Additive Explanations |
FUNDING
This study was supported by Henan Provincial Science and Technology Research Project (242102310486).
ACKNOWLEDGEMENTS
Declared none.


